What Does
Open Circuit Potential (OCP) Mean?
Open circuit potential (OCP) is defined as the potential that exists in an open circuit. That is, it is the voltage present when the terminal ends of a circuit are detached, and there is no external load.
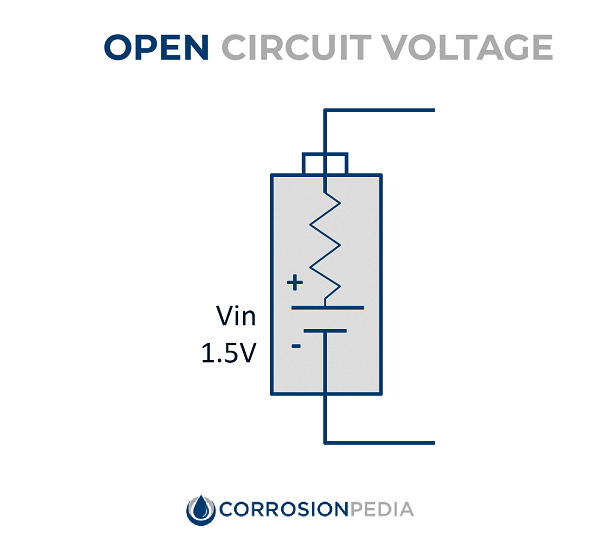
Figure 1. Figure showing an open circuit, i.e., a circuit that is not connected to form a complete electrical path.
Where the open circuit potential gives us the full potential of the battery, the same concept can be applied to electrodes in an electrochemical circuit. When an electrode is immersed in an electrolyte, it will adopt an open circuit potential. This potential ultimately defines its ability to either be oxidized or reduced.
In a typical electrochemical cell, two metals are immersed in an electrolyte, causing both of them to adopt an open circuit potential. If a voltmeter is connected to the two electrodes, their potential difference (the difference in potential between the two electrodes) can be measured.
If the two metals are electrically connected (e.g., with a cable), the difference in potential that exists between the two electrodes will result in a flow of electricity as electrons move from the metal with the lower potential to the metal with the higher potential. The material with the lower potential becomes the anode, while the material with the higher potential becomes the cathode.
An understanding of open circuit potential is crucial in the corrosion industry as it helps to predict how metallic materials will participate in electrochemical corrosion reactions in a given medium.
Open circuit potential is also known as open circuit voltage (OCV).
Corrosionpedia Explains Open Circuit Potential (OCP)
To better understand the concept of open circuit potential, consider Figure 2 (below) which shows the difference between open circuit potential and the potential that is in a closed circuit connected to a load.
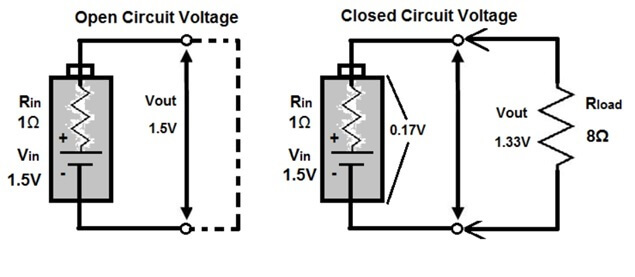
Figure 2. Open circuit voltage (left) vs closed circuit voltage (right).
In the figure to the left (which is the same as Figure 1), the voltage is measured across two points that do not form a complete electrical path. In this case, because there is no resistance in the circuit, there is no drop in voltage or potential, because the voltage does not need to be shared with a load. Therefore, the voltage measured in the open circuit is representative of the potential of the power source. If the battery has a voltage of 1.5 volts, then the potential difference measured across the two open terminals will also be 1.5 volts.
The circuit to the right, however, is closed. In other words, a complete electrical path is formed between the positive and negative ends of the battery. In addition, the circuit is also connected to a load or resistive element. In this case, the voltage in the circuit is shared among the battery and the load. Therefore, the potential difference at the battery terminal is only 0.17 volts, while the remaining 1.33 volts is dropped across the resistor.
Open Circuit Potential Versus Corrosion Potential
Despite the difference in their name, open circuit potential is the same as corrosion potential. The corrosion potential is simply the potential that an electrode has when there is no external current applied. In an electrochemical cell, current will flow from metals with a lower corrosion potential to materials with a higher corrosion potential. Therefore, a material with a naturally high corrosion potential is likely to be the cathode of the cell.