The destructive effects of corrosion on metals have been known for centuries. For almost as long as metals have been a construction material, humans have constantly sought ways to improve its longevity in corrosive environments. One of the most widely used techniques for protecting metals (mainly steel) is galvanizing.
In 1742, a chemist known as Melouin found that a zinc coating could be applied to iron by dipping it into molten zinc. This discovery triggered a wave of research throughout the scientific community and laid the foundation for galvanizing. In 1780, an Italian physicist, Luigi Galvani, the man for whom the process is named, observed that the contact between two dissimilar metals resulted in the flow of an electrical current. (For background reading, see Why Do Two Dissimilar Metals Cause Corrosion?) Over time, the understanding of galvanizing improved significantly, and by 1850, 10,000 tons of zinc was used annually by the British galvanizing industry for the protection of iron. This was the birth of an industry that continues to flourish to this day.
Galvanized steel plays an essential role in our everyday lives. It is frequently used in several sectors including construction, transportation, agriculture and power generation industries to name a few. There are two main methods for galvanizing steel; these are hot-dip galvanizing and cold galvanizing. In this article, we will look at these two galvanizing methods and discuss how these techniques differ.
What is Galvanizing?
In its simplest form, galvanizing refers to the application of a zinc coating to the surface of a metal (usually steel or iron). It is mainly used as a protective measure to prevent corrosion and, by extension, increase the serviceable life of the protected metal component. When properly applied to a steel surface, zinc coatings offer two main types of protection: barrier protection and galvanic protection.
Barrier protection
When the zinc coating is applied to the surface, it dries and hardens to form a protective zinc oxide layer that is impervious to air and moisture, thus eliminating one of the components needed for corrosion to occur – the electrolyte. Because the underlying steel substrate is unable to come into contact with external air or moisture, further corrosion is prevented.
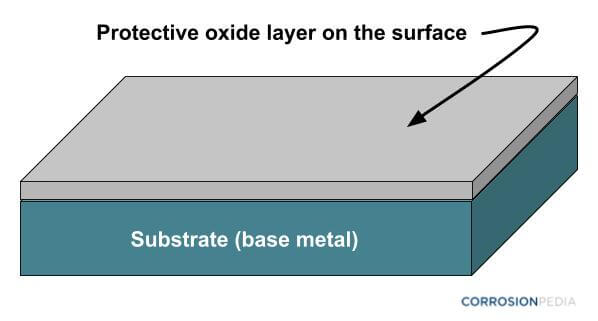
Figure 1. Zinc oxide layer provides barrier protection to the underlying substrate.
Galvanic protection
Galvanizing however, is best known for its namesake protection method. Galvanic protection, also known as cathodic protection, protects the underlying steel substrate by corroding preferentially, thus sacrificing itself in the process. This type of protection is especially useful for situations where the protected steel may become exposed due to scratches, cuts, dents or coating damage.
Because zinc is a highly reactive and electronegative metal, it will assume the role of the anode, therefore corroding first in the event that the adjacent steel is unprotected. Zinc will continue to offer galvanic protection until the coating is entirely consumed.
Hot-dip Galvanizing
Hot-dip galvanizing is one of the most common forms of galvanizing. This process entails coating an iron or steel object by immersing it into a molten zinc bath at temperatures of around 840°F (449°C). Once removed from the bath, the zinc coating on the iron or steel’s exterior reacts with oxygen in the atmosphere to form zinc oxide (ZnO).
Zinc oxide further reacts with carbon dioxide to form the protective layer known as zinc carbonate (ZnCO3). This dull grayish film is relatively stable and adheres tightly to the surface of the iron or steel. In hot-dip galvanizing, the zinc chemically bonds and becomes part of the steel being protected.
Though the concept of hot-dip galvanizing may seem simple, the process consists of several crucial steps (Figure 2). The steel first goes through three cleaning stages to prepare its surface for galvanizing; these are degreasing, pickling and fluxing. Degreasing is used to remove dirt, oil and other organic residues; a mild acid bath, or pickling, etches the steel and removes mill scale and iron oxide; and fluxing further removes any oxides and coats the steel with a protective layer that prevents the formation of further oxides on the steel surface prior to galvanizing.
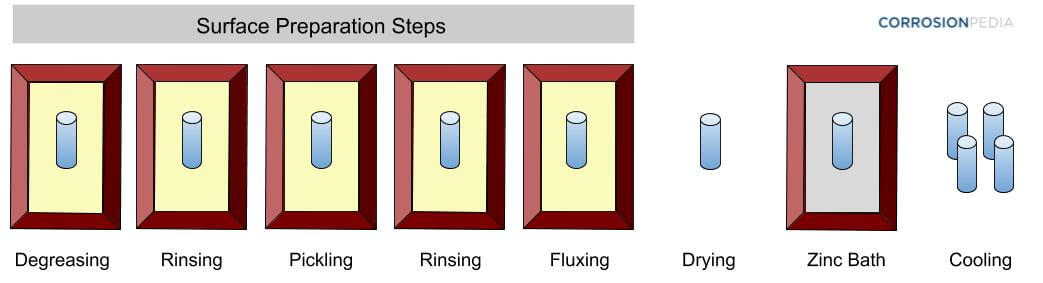
Figure 2. Hot-dip galvanizing consists of a sequence of steps.
Once the cleaning process is completed, and the structure has dried, it is ready to be immersed into the molten zinc bath. Molten zinc flows in and around the iron or steel object, thus thoroughly coating it to protect all surfaces. The coated material is then removed from the bath and air-dried before inspection.
Cold Galvanizing
Cold galvanizing is simply the application of a zinc-rich paint to the surface of a steel element to protect it from corrosion. As such, the term “cold galvanizing” is considered to be a misnomer among some professionals in the coating industry.
Zinc paints may be applied by brushes, rollers, spray guns, etc. Coatings may also be applied by the electrogalvanizing method as well. The zinc-rich paints used in cold galvanizing are different from conventional coatings due to the presence of a binding compound. These binders allow the zinc to mechanically bond to the steel to offer an effective level of protection.
Like hot-dip galvanizing, cold galvanizing can provide barrier protection and also some degree of cathodic protection. However, the zinc dust present in the paint or coating must be in high enough concentrations to promote electrical conductivity between the steel and the zinc.
Figure 3. Video describing cold galvanizing.
The surface preparation required for applying zinc-rich coatings is less demanding than hot-dip techniques. Before coatings operations begin, the surface of the steel must be clean and dry. Usually, a wire brush is first used to remove rust or any other corrosion products that may be present. Dirt, grease, chemicals and other organic compounds must also be removed accordingly. Once the surface is prepared, the zinc coating is applied to the surface in as many coats as required.
Comparing Hot-dip and Cold Galvanizing
Although hot-dip and cold galvanizing both serve similar purposes, their method of application and performance differ significantly. Cold galvanizing, unfortunately, does not offer the same level of protection as its hot-dip counterpart. Because cold galvanizing is simply a coating, it cannot bond with the metal on a chemical level and, as such, does not have the same durability, abrasion resistance and cathodic protection capabilities as hot-dip galvanizing.
While cold galvanizing does not live up to the performance of hot-dip galvanizing, it does have its benefits. Cold galvanizing is ideal for cost-effective and rapid application on smaller structures and components. Hot-dip processes are more expensive and better suited for larger structures, typically for heavy-duty industrial uses. (Related reading: Galvanization and its Efficacy in Corrosion Prevention.)
The choice of galvanizing method ultimately boils down to finding the right balance between cost and coating performance for a given application.