Analysis and identification of corrosion products is a critical component of a failure analysis in order to conclude the root cause and to determine the corrective action that is required to prevent future occurrences. (For background reading on conducting a failure analysis, see The 3 Stages of Corrosion Failure Analysis.) However, the analysis of any sample is only as good as what is being analyzed because many oxide and sulfide corrosion products are very susceptible to degradation upon atmospheric exposure.
Often the analyst uses a scanning electron microscope (SEM) to identify the elements present in the corrosion products, but there are many other instrumental techniques such as x-ray diffraction (XRD) that can be employed to provide vital information. In this article the applicability of various analytical tools will be discussed.
Using Energy Dispersive X-ray Spectroscopy (EDS) to Analyze Corrosion Products
Energy dispersive x-ray spectroscopy (EDS) associated with a scanning electron microscope (SEM) is the most common technique used to obtain chemical identification of corrosion products. EDS can detect elements C through U with a detectability limit of about 0.1 weight percent. The scanned electron beam interacts with the specimen's surface, which produces secondary and back-scattered electrons and x-rays. These x-rays are characteristic for the element that emits them and can be used to identify the elemental composition of corrosion products. Unless standards are used, the analysis produces semi-quantitative analysis based on the computer software and assumed instrumental parameters.
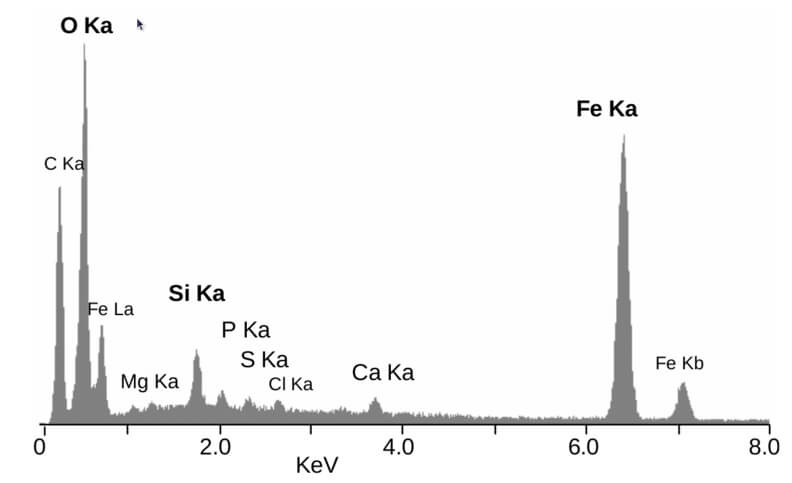
Figure 1. Elemental energy dispersive X-ray microanalyses from a mineral particle ~2 μm diameter. The peaks are labelled with the line of the corresponding element. (Source: Creative Commons.)
Often the initial EDS analysis is of the corrosion product on a metal or fracture surface. This can provide information as to which metals (C, O, F, S or Cl) are present; but the exact composition of the corrosion product cannot be concluded. Although the x-rays that are analyzed come from the surface, they are emitted from an excluded volume that can be several microns deep. This excluded volume can include the base metal or multiple layers of the corrosion scale. (Related reading: Corrosion Scale Analysis for Piping Specimens.)
Removal of the scale from the base metal or fracture surface can provide a more realistic analysis of the corrosion product chemistry. The use of acetone-softened cellulose acetate replicating tape is an excellent technique to remove the scale from the base metal. If the tape procedure is used, an unused portion should be analyzed to identify potential contaminant elements from the tape that are not in the scale. Also, if a C or Au coating is used to provide electrical conductivity, the low energy x-rays will be suppressed and any data reduction can be skewed.
Another issue with EDS analyses is the requirement of a sufficient accelerating voltage to excite the requisite x-rays. In addition, there can be peak overlap that can make data interpretation difficult and force reliance on software for differentiating; for example, the Mo-La from S-Ka peaks directly overlap and identifying the presence of either or both can be compromised. Increasing the accelerating voltage to include the Mo-Ka or using a wavelength dispersive x-ray spectrometer can be used to positively identify the presence of Mo and/or S.
Many corrosion scales consist of layers of different compositions such as high temperature sulfide scale on austenitic stainless steel. Thus, analysis of the scale from the exterior surface will not provide sufficient detail. A metallographic cross-sectional analysis or a focused ion beam prepared section with subsequent EDS analysis is the best approach for identification of element location.
Using X-ray Diffraction (XRD) to Analyze Corrosion Products
EDS analysis provides elemental information such as Fe and O, but cannot differentiate whether the corrosion products are hematite or magnetite. Such identification can be done with x-ray powder diffraction. With this technique the angles and intensities of x-rays diffracted from the planes of the crystalline structure are unique, allowing for the identification of the various compounds in the corrosion scale.
Elemental analysis via x-ray diffraction (XRD) is very useful for identification of compounds present because atomic substitution will produce a small lattice strain that can be difficult to detect. For example, substitution of Ca+2 ions into FeCO3 may not be detectable. Because the shape of crystals of corrosion products/scale tends to be platy, fibrous or tabular, they may not randomly orient during sample preparation, which can cause some planes to produce a greater intensity ratio than others and impact the quantitation from peak intensity ratios. Quantification of the various components requires a Rietveld refinement where a least squares analysis is used to refine a theoretical pattern until it matches with the measured pattern.
When the sample quantity is small, dispersing the corrosion products onto a glass slide can often produce a satisfactory diffraction pattern. Combining micro-focused x-ray diffraction with SEM can provide both elemental and phase identification of the exact same location. Such an approach can be quite useful for multi-colored scales and spatial distribution analyses.
Bulk Analysis of Corrosion Products
Although an EDS analysis can provide local elemental analyses, it is sometimes quite useful to obtain a bulk quantitative analysis of the scale. Such information can be used to identify and quantify trace elements or the potential metal source for the deposit if it was no longer adherent to the metal. Potential instrumental techniques include x-ray fluorescence (XRF), inductively coupled plasma (ICP-MS) and atomic adsorption spectroscopies. Various chromatographic instrumentation can be used to detect and quantify ion constituents in corrosion deposits such as Cl–, F–, CO3=, and NH4+. Special sample preparation is required for these techniques.
Surface Analysis of Corrosion Products
Specialized analyses can provide additional insights into corrosion products. Surface analysis techniques such as Auger electron spectroscopy (AES) or x-ray photoelectron spectroscopy (XPS) can provide information on the outer atomic layers. In addition, XPS can determine the binding energy of elements present on the surface, which can provide information on their chemical state. These techniques are extremely useful for analyzing thin protective scales, multi-layer deposits and sub-surface diffusion into the base metal. Depth profiling is a useful XPS technique, but interpretation can be muddled by ion mixing from the sputtering process.
Fourier transform infrared spectroscopy (FTIR) and Raman imaging and spectroscopy are often used to identify organic compounds in corrosion products. Both techniques can analyze micro-volumes.
Raman spectroscopy is a light scattering technique where the molecule scatters the incident high intensity laser light based on the chemical structure. This can be useful for determining some surface species because the analysis does not require a vacuum. For example, identification of amorphous versus graphitic ordered carbon deposits by Raman can be used to determine the type of coke and how it was formed, which can be critical for continued operation of a refinery or petrochemical plant.
Raman can also be used to differentiate the various surface iron oxides, hydroxides and oxyhydroxides, aluminum oxides, copper corrosion products and zirconium oxides on a metal surface. Note, however, that not all metal oxides or sulfides have the necessary molecular vibrations or excitations for detection.