The anodizing process enables the formation of a brilliant, stable and durable oxide layer upon certain metals, which minimizes wear and corrosion damage to the underlying metallic substrate. The thick anodic oxide layer also serves as an effective base for applying an additional colored coating layer to further enhance the surface protection, luster and aesthetics of a substrate.
Here we'll take a look at anodizing, how it works and why it's the preferred metal finishing process for aluminum, titanium and similar metals and alloys
The Anodizing Process
Anodizing involves an electrochemical process that enhances the ability of metallic surfaces to absorb oxygen by having the surface immersed in an acid solution and connecting a voltage source across the metallic object to be anodized.
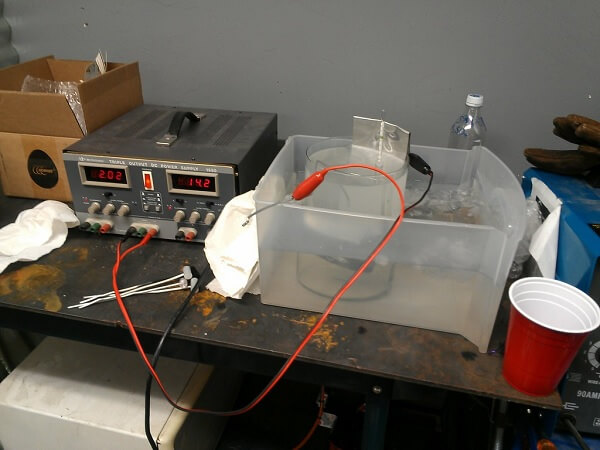 A demonstration of anodization
A demonstration of anodization
Source: Jasper Nance
Thus, the anodic oxidation of metals such as aluminum, zinc, cadmium, magnesium and titanium and their alloys enables the creation of a hard layer of their respective metallic oxide (e.g., aluminum oxide, magnesium oxide, titanium oxide, etc.). These stable oxides strongly adhere to the metal substrate without the tendency to flake or fall off that is observed in the case of ferrous surfaces that become rusted during oxidation. (For an introduction to anodizing and other methods, read The 5 Most Common Types of Metal Coatings that Everyone Should Know About.)
Classifications of Anodizing
There are several anodizing classifications:
Hard anodizing
The hard anodization process enables the formation of an oxide film of greater thickness, which is generally on the order of twenty micrometers to a hundred (or more) micrometers. The higher oxide film thickness is achieved by increasing the DC voltage and the concentration of the acid, while maintaining the bath at lower temperatures.
Hard anodizing creates a superior corrosion resistant layer that is hard as well as abrasion resistant. For example, aluminum anodized in a solution of sulfuric acid at a temperature of 5°C (41°F) creates a thick layer of hard anodic oxide with a dull grey color, whereas at a bath temperature of 20°C (68°F) the sulfuric acid solution creates a soft and thin anodic film.
Sulfuric acid anodizing
The process of sulfuric acid anodizing enables the formation of precisely controlled thicknesses of anodic oxide films on the metallic substrate. The desired color finish is obtained through an additional color treatment. The precise thickness is achieved through the choice of voltage, bath temperature and the composition of the acid solution. For aluminum anodization, a sulfuric acid bath solution is commonly used.
Chromic acid anodizing
Chromic acid anodizing is the main choice if there is a requirement that the overall fatigue strength of the product not decrease due to the process. The anodization layer's thickness is between one to 10 micrometers. This is not a preferred method if the color consistency of the outer surface is important, because a very thin oxide film cannot be the base for consistent coloring.
Aluminum alloys that are used for their high strength in aircraft are often anodized by chromic acid anodizing. However, the process is not environmentally friendly because it contains chromium (VI), whose usage is restricted by regulations due to its toxicity.
White anodizing
The white anodizing process has been studied for its suitability in space applications because it produces an oxide film with a low solar absorptance value. In this type of anodizing, the solution consists of sodium molybdate, glycerol, lactic acid and sulfuric acid. Optimum film thickness and optical color consistency are achieved by studying the impact of alternative formulations of bath solutions, DC voltage, current density, temperature of the bath and the duration of anodizing.
Silicon anodizing
When the alloy contains silicon, the resulting layer is more resistant to wear and corrosion, athough it has a characteristic grey and opaque color. This type of anodizing is not used for decoration, but rather for parts that will not be visible.
Titanium anodizing
Titanium anodization is carried out in a diluted bath of sulfuric acid while applying a fixed value of DC voltage. The impact of process variables such as process duration, acid solution formulation, bath temperature and current density on the anodic oxide film thickness and color properties have been studied to optimize the titanium anodization process. (Related reading: 5 Things to Know and Understand About Titanium Corrosion.)
Orthopedic titanium alloy anodization
Anodizing is one method of forming a nano-structure anodic oxide film on the surfaces of titanium alloys used for biomedical implant applications. In this process, the fine adjustment of oxide layer thickness and other characteristics such as topography of the pores constituting the layer is possible.
Titanium alloy based orthopedic implants are color coded through anodization in a sulfuric acid solution. One study indicated that if the color-coded implant is anodized again in a hydrofluoric acid solution, the implant would potentially facilitate increased bone growth in the patient.
Anodized standard color coding of implants and devices used in dental, orthopedic and other applications facilitates quick recognition, accurate and fast assembly of components and eases medical procedures. This advantage is applicable to the assembly of anodized (color-coded) titanium alloy components used for aerospace applications as well.
Magnesium anodizing
Magnesium anodization is carried out in an alkali rich electrolyte. The bath formulation ensures that the film formed on the surface has high corrosion resistance, saltwater resistance, wear resistance and an aesthetic finish.
The formation of the anodic oxide film of magnesium is directly affected by the voltage. Anodizing magnesium at a low applied voltage does not enable an oxide film with adequate corrosion protection, thus higher DC voltages are necessary. New anodizing processes have adopted spark discharge energy to produce a wear-resistant ceramic oxide film on magnesium substrates. (Ceramic coatings are discussed in the article Top 5 Applications for Ceramic Coatings.)
The Chemistry of Anodizing
During the process of anodizing, a DC voltage is applied between a metal workpiece (e.g., aluminum) and a metallic cathode (often zinc is used as a cathode). The water particles of the acid solution break down near the anode, generating oxygen that is collected at the anode. Rich oxygen reacts with the aluminum to produce aluminum oxide (Al2O3).
2Al + 3H2O à Al2O3 + 6H+ + 6e–
A thin layer of aluminum oxide is quickly formed on the substrate and a thicker oxide layer of porous structure is formed at a slower pace. On the surface of aluminum a thin layer of anodic oxide may already be present, but this thin layer is susceptible to damage and cannot ensure strong corrosion and abrasion resistance.
Anodizing enhances the thickness and other characteristics of the anodic oxide film according to the requirements. These anodic oxide film parameters can be tailored to the specific service conditions (such as found in the chemical industry or in proximity to coastal areas). Whenever the oxide film's porosity is not acceptable, a non-porous film can be created by anodizing in a non-acidic neutral bath.
Pre-process Treatment
Pretreatment prior to anodizing includes thorough cleaning and etching. As the workpieces may be received in soiled condition, a proper cleaning is required. Etching can be done in a solution of sodium hydroxide. Properly etched surfaces will not reveal surface defects such as scratches after the anodization.
Post-Processing Coloring and Sealing
In the case of magnesium, the anodizing is often used as preparation for a subsequent coloring or painting process. Often dyes are used for coloring anodized surfaces and a coating of polytetrafluoroethylene (PTFE) is used to improve wear resistance and reduce friction. Coloring is used to facilitate assured identification as well as improve aesthetics.
A subsequent sealing process plugs the pores, thus contributing to the stability of the anodic oxide film so it can resist wear and tear as well as corrosion associated with saltwater splashing and the deep ocean environment.
In the case of titanium anodizing, however, there is no separate coloring process because color finishing is achieved by directly fine-tuning the anodization process parameters.
For aluminum anodizing, the workpiece is cleaned and etched before being placed in an acid solution in an anodizing tank. It is connected as the anode and the negative terminal is connected to cathode plates (or rods) in the electric circuit. Current flow in the circuit causes the aluminum substrate to react with oxygen released from water to produce aluminum oxide, which strongly adheres to the substrate. Pores of anodic aluminum oxide are formed deep in the surface, creating a strong barrier film that protects the surface from corrosive environments. As long as voltage is applied across the terminals of the circuit, the oxygen continues to penetrate and oxidize the aluminum, thus creating a thicker and stronger barrier film. Once the designed film thickness is achieved, power is switched off.
If coloring is needed, dye is prepared in a separate vessel, and the anodized workpiece is placed in the vessel after a water rinse. After coloring, the anodized and dyed workpiece is placed in hot water for sealing. The sealing process adds to the metallic sheen and durability of the aesthetic coloring. As the surface is etched, the rays of light falling on the colored surface are reflected partly by the uncolored pores and partly by the colored pores, thus maintaining the durable metallic luster of the coloring used. This is why anodized aluminum is so popular for decorative applications.
Anodized titanium is used in medical devices and aerospace applications. The advantage of anodizing this metal is that it will not alter the mechanical properties of the bulk metal. Anodizing facilitates easy identification of the parts and components during assembly and subsequent use as well.
Anodized aluminum is suitable for applications near a marine environment, for window frames and the fascia of large buildings and commercial complexes. For decorative and aesthetic purposes the oxide film should be transparent and not grayish. The bath temperature must be controlled wherever a decorative finish is desired.
Anodized metals are also used for:
- Aesthetic ornaments, artwork, architectural structures and parts
- Automobile and aircraft components
- Luxury furniture, sporting equipment
- Kitchen appliances, food manufacturing machine components
- Components used in building construction
Anodizing Equipment
The DC power needed for the anodizing process is supplied through rectifiers. Years ago, motor-generator sets (MG sets) were used to convert AC power to DC. The voltage needed could vary from 24 to 70 volt DC. Modern power equipment is able to supply pulsed current, which is needed to produce anodic film with higher corrosion resistance. One manufacturer claims that pulsed current (with microprocessor-based control) increases the production rate with higher current densities while keeping surface temperatures lower, thus reducing the load on refrigeration requirements.
Temperature control equipment requires a refrigeration system because the anodizing process produces heat energy (exothermic electrochemical reaction) that must be absorbed without causing the bath temperature to rise.
The electrolyte is agitated by an air blowing system so that the entire bath has a uniform temperature. Extraction equipment fitted to the anodizing tank removes the hydrogen and acid mist that is continuously produced near the cathode.
Anodization tanks can be used as the cathode if they are lined with lead. Most often, separate cathodes are positioned all along the length of the tank because controlling the anode area to cathode area ratio is critical in some types of anodizing. For a sulfuric acid bath, an aluminum cathode has advantages over a lead electrode. Tanks made of steel and lined with neoprene rubber or acid resistant polymers are commonly preferred for this application.
Anodizing Definitions and Methods
While the chemical anodizing procedure is same across all applications, the mechanical processes differ depending on the physical types and shapes of metals used:
Batch anodizing entails submerging racking pieces in a series of treatment tanks. Batch anodized objects include extrusions, sheets or bent metal pieces, castings, cookware, cosmetic cases, flashlight bodies, and machined aluminium parts, to name a few.
Continuous coil anodizing involves continuously unwinding pre-rolled coils and passing them through a sequence of anodizing, etching and cleaning tanks before rewinding them for shipment and fabrication. This technology is used to make lighting fixtures, reflectors, louvres, spacer bars for insulated glass, and continuous roofing systems out of high volume sheet, foil and less severely shaped goods.
Conclusion
Anodization is a metal finishing process during which a metallic workpiece is connected as the anode and immersed in an electrolyte of a chemical (acidic) solution in order to form an anodic oxide film on its surface. This film is stable, resistant to abrasion and corrosion, and also works as a base for any further coloring needed for identification or aesthetic purposes.
Oxide film parameters are influenced by process variables such as applied DC voltage, the duration of the process, electrolyte chemistry and the bath temperature. While anodized titanium components are used for orthopedic implants, both anodized aluminum and titanium parts are used in critical aerospace applications. Additionally, anodized metals are used for numerous industrial and architectural purposes because they are elegant, durable and weather resistant.