Corrosion is a naturally destructive phenomenon that occurs when some metals are exposed to the environment. The reaction between air, moisture and the metal substrate gives rise to specific chemical reactions that cause the metal to convert to its more chemically-stable oxide, hydroxide or sulfide form. In iron-based metals, such as steel, corrosion comes in the form of iron III oxides, also known as rust.
For electrochemical corrosion to occur, three ingredients must be present: an anode, a cathode and an electrolyte. The anode and cathode are usually connected via a continuous electrical path while both are immersed in the same electrolyte. During this process, the anode experiences corrosion, while the cathode remains unaffected.
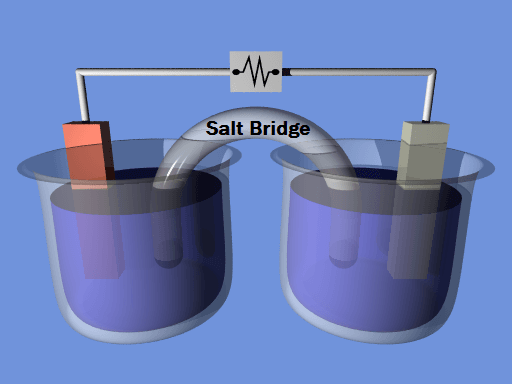
Figure 1. A typical electrochemical cell showing electrons flowing from the anode to the cathode through an electrical connection. (Source: Alksub at the English Wikipedia / CC BY-SA)
There are various methods to prevent and control corrosion. One of these is known as cathodic protection (CP). This technique works by connecting the metal to be protected to a more easily corroded "sacrificial metal." This sacrificial metal corrodes preferentially (acting as the anode) while the more valuable metal object under consideration (acting as the cathode) remains protected. In this article, we will explain how this sacrificial protection method works and describe its various applications.
To understand how cathodic protection works, we must first appreciate the basics of bimetallic corrosion, also known as galvanic corrosion. Bimetallic corrosion, as its name implies, is a unique type of corrosion that occurs between the pairing of two metals. This corrosion is observed in several situations where dissimilar metals are in direct or indirect contact with each other. Bimetallic corrosion is usually characterized by accelerated corrosion in one metal while the other remains unaffected. In other words, one metal sacrifices itself while protecting the other. (This process is examined more fully in the article Why Do Two Dissimilar Metals Cause Corrosion?)
Corrosion in an electrochemical cell is driven mainly by a property known as potential difference. This potential difference causes electrons to flow from one metal in the cell (the anode) to the other metal (the cathode) while generating a small amount of electricity in the process. As electrons flow out of the anode, oxidation occurs, causing the anodic metal to degrade or corrode. Meanwhile, as electrons flow to the cathode, reduction occurs, further protecting the cathodic metal.
In bimetallic corrosion, this potential difference is a direct result of the difference in electrode potential between the two dissimilar metals. When a metal is immersed in an electrolyte, it adopts an electrode potential that represents the metal’s ability to be oxidized or reduced. The electrode potential of various metals is displayed on a list known as the galvanic series. (See An Introduction to the Galvanic Series: Galvanic Compatibility and Corrosion for more information.) The metals positioned higher on the table are considered to be anodic (more electronegative), while the metals placed lower on the table are more cathodic (more electropositive). The further apart the contacting metals are in the galvanic series, the greater the potential difference between the metals, thus the more severe the corrosion at the anode.
Cathodic Protection (CP) and Its Method of Operation
While the design of cathodic protection systems can be sophisticated, their operation is based on the concept of bimetallic or galvanic corrosion described earlier. By understanding the principles of this type of corrosion, we can purposely pair metals together to ensure that one cathodically protects the other. In other words, if we want to protect a particular metallic structure, we can create conditions where this metal becomes the cathode of an electrochemical cell. By electrically connecting the metal to be protected to a more anodic (electronegative) metal, we can ensure that the anode sacrifices itself by corroding preferentially over its cathodic counterpart.
In some cases, external power sources can be used to supply additional electrons to the electrochemical process, which can increase the effectiveness of cathodic protection.
Cathodic protection systems are employed in numerous industries to protect a broad range of structures in challenging or aggressive environments. The oil and gas industry, in particular, uses cathodic protection systems to prevent corrosion in fuel pipelines, steel storage tanks, offshore platforms, and oil well casings. In the marine industry, this protection method is also used on steel piles, piers, jetties and ship hulls. Another common type of cathodic protection, known as galvanizing, is commonly used to protect steel members and structures. (To learn more, read Galvanization and its Efficacy in Corrosion Prevention.)
Types of Cathodic Protection (CP)
As mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. This can be achieved by employing two distinct types of cathodic protection: passive cathodic protection and impressed current cathodic protection.
Passive cathodic protection
In passive cathodic protection systems, the sacrificial anode is connected directly or indirectly to the metal to be protected. The potential difference between the two dissimilar metals generates adequate electricity to form an electrochemical cell and drive galvanic or bimetallic corrosion.
This type of protection is commonly used in the oil and gas industry to protect the structural steel members of offshore rigs and platforms. Here, aluminum bars (or another suitable metal) are mounted directly on steel sections to assume the role of the sacrificial metal. Steel water heaters, tanks and piles are also cathodically protected using a similar method.
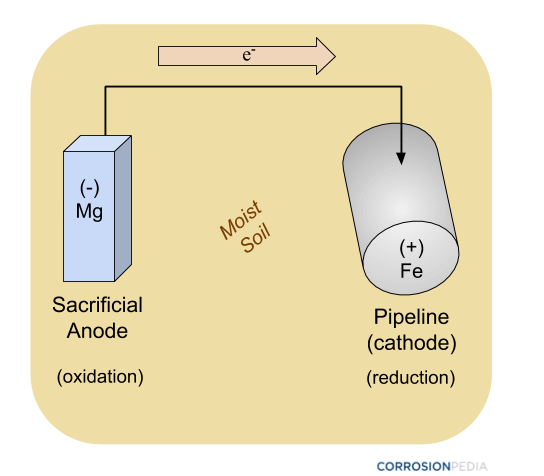
Figure 2. Schematic of a pipeline being protected by a sacrificial anode using passive cathodic protection methods. Notice how there is no external power source involved.
Another common example of passive cathodic protection is hot-dipped galvanized steel. During this process, steel members or structures are immersed in a molten zinc bath that coats the object. When the steel is removed from the molten zinc, it reacts with air and moisture to form a protective layer known as zinc carbonate, which creates a galvanic cell with the steel.
When the steel member is scratched or damaged, such that the substrate is exposed, the surrounding zinc coating acts as the sacrificial anode and corrodes preferentially to protect the exposed steel. This type of protection continues until the nearby zinc is depleted.
Impressed current cathodic protection (ICCP)
In large structures, it may not be feasible to use passive cathodic protection methods. The number of sacrificial anodes required to deliver enough current to provide adequate protection can either be unrealistic or impractical. To address this, an external power source is used to assist in driving the electrochemical reactions. This technique is known as impressed current cathodic protection (ICCP). ICCP systems are ideal for protecting lengthy structures, such as underground pipelines. The flanges of connecting pipes are usually insulated using isolation kits to separate the pipes into smaller, more manageable sections for the purposes of ICCP protection.
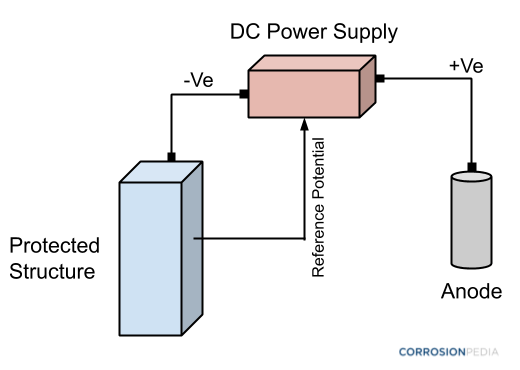
Figure 3. Schematic of an object being protected by an anode using impressed current cathodic protection (ICCP) methods. Notice how an external DC power source is involved.
Limitations of Cathodic Protection
In large pipeline networks, there may be many crossings, parallelism and approaches near the pipeline’s CP system. DC interference may occur between pipelines, which accelerates corrosion. In order to overcome this problem, pipelines can be electrically coupled, either directly or through resistance.
For coated pipelines, cathodic disbondment may occur due to high CP levels where the applied coating quality is poor. Higher temperatures may also promote cathodic disbondment. High pH environments are also a concern in terms of stress-corrosion cracking.
Conclusion
Cathodic protection is a popular protection method for preventing corrosion in pipelines, offshore oil platforms and other steel structures. However, to be implemented effectively, it is crucial to understand the basic principles of bimetallic/galvanic corrosion. Choosing the right type of cathodic protection system depends on several factors, including cost-effectiveness and the size of the structure to be protected.