Corrosion is an electrochemical method by which materials are deteriorated. In many cases—and especially when liquids are present—it involves chemistry. During corrosion, electrons from distinct areas of a metal surface flow to alternative areas through an atmosphere capable of conducting ions. That's the simple chemistry of corrosion, but the details are anything but. The same goes for the impact.
In fact, the economic impact of corrosion is much bigger than many realize. According to a 2001 report by CC Technologies Laboratories Inc., the cost of corrosion within the U.S. alone was $276 billion annually. Of this, $121 billion was spent to manage corrosion, while the remaining $155 billion was incurred as a net loss to the economy. Utilities, particularly water and sewer systems, suffer the biggest economic impact, with motorized vehicles and transportation coming in a close second.
Because metallic corrosion is an ongoing electrochemical process, it's crucial to know the essential nature of electrochemical reactions to properly inhibit corrosion and reduce its impact on structures. In this article, we'll discuss the mechanisms of corrosion by covering the details of:
- Electrochemical reactions
- The Daniell cell
- The anodic method
- Faraday's law
- The cathodic method
- Surface area impact
What is Corrosion Electrochemistry?
Corrosion in an aqueous environment and in an atmospheric setting is an electrochemical process in which electrons are transferred between a metal surface and a liquid electrolyte solution, resulting in the deterioration of the substrate. Corrosion occurs because of the great tendency of metals to react electrochemically with oxygen, water and alternative substances within the atmosphere. In this context, the term anode is employed to explain the portion of the metal surface that's really corroding, whereas the term cathode is employed to explain the metal surface that consumes the electrons created by the corrosion reaction. Ulick R. Evans, an early pioneer in explaining corrosion as an electrochemical process, said that it could be described as destruction by electrochemical or chemical agencies. Corrosion electrochemistry, therefore, is simply an electrochemical method through which we can perceive the mechanisms of corrosion.
Electrochemical Reactions
An electrochemical reaction is outlined as a reaction involving the transfer of electrons. It's also a reaction that involves oxidation and reduction. The very fact that corrosion consists of a minimum of one chemical reaction and one reduction reaction isn't entirely obvious because both reactions are usually combined in one piece of metal (e.g., zinc), as illustrated schematically below.
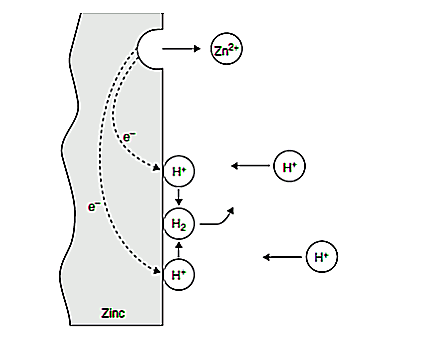
Figure 1. Electrochemical reactions during the corrosion of Zn in air-free HCL.
In Figure 1, a bit of zinc immersed in acid solution is undergoing corrosion. At some point on the surface, Zn is transformed to Zn ions losing electrons. These electrons go through the solid conducting metal to alternative sites on the metal surface, wherever hydrogen (H) ions are reduced to hydrogen gas consistent with the following equation:
 The Daniell Cell and Electrochemical Corrosion
The Daniell Cell and Electrochemical Corrosion
The doctrine of electrochemical reactions is employed in a Daniell cell, during which copper and zinc metals are immersed in solutions of their individual sulfates. The Daniell cell was the primary sensible and reliable battery that supported several 19th-century electrical innovations, such as the telegraph.
In a Daniell cell, electrons are transferred from the corroding zinc to the copper through an electrically conducting path as an electric current. (Learn about a similar corrosion cell in the article An Introduction to the Alexander Cell.) Zinc loses electrons more readily than copper, which means that putting zinc and copper metal in solutions of their salts will cause electrons to flow through an external wire that leads from the zinc to the copper as per the following reactions:
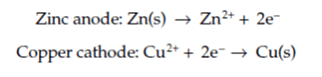 corrosion potential between the two metals will usually cause a scenario that's referred to as galvanic corrosion, which was named in honor of its discoverer, Luigi Galvani.
corrosion potential between the two metals will usually cause a scenario that's referred to as galvanic corrosion, which was named in honor of its discoverer, Luigi Galvani.
This situation is common in natural corrosion cells wherever the setting is the electrolyte that completes the corrosion cell. The conduction of a liquid atmosphere like soil, concrete, or water has usually been associated with its corrosivity.
The short-hand description within the following equation is valid for each Daniell cell configuration.
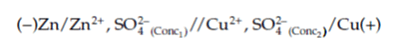
This equation identifies the zinc electrode as the anode because it is negative in the case of a spontaneous reaction, while the copper electrode is the cathode due to its positive charge.
The Anodic Method and Corrosion
Now we move into more detail about what takes place at the anode once corrosion occurs (anodic method). For example, the corrosion reaction for iron (Fe), which involves the reduction of hydrogen ions to hydrogen gas, is consistent with the electrochemical reaction of zinc in hydrogen chloride (HCl). This hydrogen evolution reaction happens in a variety of metals and acids, and may involve hydrochloric, sulfuric, perchloric, hydrofluoric, formic, and alternative acids. The individual anodic reactions for iron, nickel, and aluminum are listed as follows:
 valence charge of n+ and therefore the release of "n" electrons. The worth of n, of course, depends totally on the character of the metal. Some metals, like silver, are univalent, whereas multivalent iron, titanium, and uranium possess positive charges as high as +6. This equation is a general one and it applies to any and all corrosion reactions.
valence charge of n+ and therefore the release of "n" electrons. The worth of n, of course, depends totally on the character of the metal. Some metals, like silver, are univalent, whereas multivalent iron, titanium, and uranium possess positive charges as high as +6. This equation is a general one and it applies to any and all corrosion reactions.
Faraday's Law and Corrosion Electrochemistry
If the current generated by one of the anodic reactions expressed earlier was familiar, it'd be attainable to convert this current to a similar mass loss or corrosion penetration rate using a helpful relation discovered by Michael Faraday. (See Corrosion Rate Conversion: Simple Ways to Convert Data Between Common Corrosion Units to learn about corrosion rates.) Faraday's empirical laws of electrolysis relate the current of an electrochemical reaction to the quantity of moles of the element being reacted. Supposing that the charge needed for such a reaction was one electron per molecule, as is the case for the plating or the corrosion attack of silver, it can be shown as:
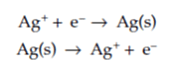 stoichiometry gives us the following equation:
stoichiometry gives us the following equation:

Where:
N is the number of moles and ΔN is the change in that quantity
n is the number of electrons per molecule of the species being reacted
I is the total current in amperes (A)
t is the period of the electrochemical method in seconds (s)
The Cathodic Method
When hydrogen (H) ions are reduced to their atomic type, they typically mix, as shown earlier, to provide hydrogen gas through a reaction with electrons at a cathodic surface. This reduction of hydrogen ions at a cathodic surface can disturb the balance between the acidic hydrogen (H+) ions and the base-forming hydroxyl (OH-) ions, making the solution less acidic, or more alkaline or basic in this region.
In neutral water, the anodic corrosion of some metals, like alumunim (Al), zinc (Zn) or magnesium (Mg), creates enough energy to separate water directly, as illustrated within the following equation and figure:

Figure 2. Electrochemical reactions of Mg during corrosion in neutral water.
The change in the concentration of H ions, or the increase in hydroxyl (OH) ions, may be shown by testing pH levels to find surfaces on which cathodic reactions are taking place. There can be many cathodic reactions encountered throughout the corrosion process. They include the following:
Oxygen Reduction

Oxygen reduction is a common cathodic reaction because oxygen exists within the atmosphere and in solutions exposed to the environment. Although not frequent, metal ion reduction and metal deposition will cause severe corrosion problems, for instance: the plating of copper ions, which are created upstream in a water circuit, on the inner aluminum surface of a radiator. Therefore, the use of a copper conduit in a water-based circuit where aluminum is also present should generally be avoided.
All corrosion reactions are merely combinations of one or many of the above cathodic reactions in conjunction with an anodic reaction. Thus, each case of liquid corrosion may be reduced to those equations in most cases, either on an individual basis or in combination. Take into account the corrosion of Zn (zinc) by water or wet air. By multiplying the Zn oxidization reaction by 2 and summing this with the oxygen reduction reaction, one obtains the following equation:

The products of this reaction are Zn2+ and OH–, which at once react to make insoluble Zn(OH)2. Likewise, the corrosion of Zn by copper sulfate represented within the following equation is simply the summation of the oxidization reaction for Zn and the metal deposition reaction involving copper (II) ions:

During corrosion, more than one oxidation and one reduction reaction might take place. In the corrosion of Zn in a concentrated HCL solution containing dissolved oxygen, for example, two cathodic reactions are possible. One is an evolution of H, while the other is the reduction of oxygen. Because there are two cathodic reactions or methods that consume electrons, the general corrosion rate of zinc is overstated. Thus, it is typically more corrosive than air-free acids, and removing oxygen from acid solutions can typically make these solutions less corrosive. This is a typical method for reducing corrosivity in many settings. Oxygen can be removed by either chemical or mechanical means.
Surface Area Impact
In corroding a piece of metal, the electrons created at anodic areas flow through the metal to react at cathodic areas that are equally exposed to the environment where they restore the electrical balance of the system. The very fact that there is no net accumulation of charges on a corrosion surface is vital for understanding most corrosion processes and ways to mitigate them. However, the equality between the anodic and cathodic currents expressed within the following equation doesn't mean that the current densities for these currents are equal:
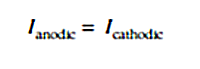
By taking the relative anodic (Sa) and cathodic (Sc) surface areas (and their associated current densities ia and ic expressed in units of mA/cm2), this equation can be expressed in terms of current densities.

The importance of the surface area ratio (Sc/Sa) of the above equation is notably vital when it comes to several varieties of localized corrosion, such as pitting and stress corrosion cracking. (For more about stress corrosion cracking, see What Causes Stress Corrosion Cracking in Pipelines?) It has great implications for the occurrence of corrosion in dissimilar metals as well.
It is simple to know that the result of a particular quantity of anodic current focused on a small area of a metal surface will be much bigger than when the same quantity of current is dissipated over a much larger area. This is an important amplifying issue of the anodic current when Sc/Sa is greater than one and a stifling factor when it is less than one.
The cause of current density may be seen when two dissimilar metals are joined (here it's Cu and Fe), which are shown diagrammatically in Figure 3 below.

Figure 3. The areas affected by dissimilar metals, where "a" shows rivets of steel on copper plates, while "b" shows rivets of copper on steel plates.
When steel rivets are part of copper plates, the corrosion of the cathodic copper plates will be low, while the corrosion of the small anodal steel rivets will be high. On the other hand, if copper rivets are joining steel plates, corrosion on the copper will be high, while corrosion of the steel plates will hardly be noticeable.
The Key to Understanding Corrosion Prevention
In this article, we examined corrosion as an electrochemical phenomenon by which materials/structures are deteriorated. Electrochemical reactions of corrosion are discussed in detail with the help of the Daniell cell, the anodic method, the cathodic method, Faraday's laws and surface area effects. Because we can prevent corrosion if we thoroughly understand the electrochemistry of corrosion, manufacturers and users of corrosion products should be attentive to the electrochemical mechanisms behind corrosion.