Corrosion costs the global economy around $2.2 trillion each year, according to the World Corrosion Organization. Of that, it is estimated that 40 to 60 percent of pipe maintenance costs are due to corrosion under insulation (CUI).
Corrosion under insulation is a hidden phenomenon. For practical reasons, it may not always be possible to apply the best insulation-coating combination or use the most feasible inspection methods. (For a rundown on inspection methods, read The 4 Best Nondestructive Inspection Methods for Corrosion Under Insulation.)
Intruding water is the key problem causing CUI. Corrosion may attack the jacketing, the insulation hardware or the underlying piping or equipment. The corrosion may present itself in one of several different types, such as chloride, galvanic, acidic or alkaline corrosion. By understanding the types of corrosion that may occur under insulation, the proper materials and construction methods can be proactively applied to prevent them.
Environmental Conditions Leading to Corrosion Under Insulation
Foreseeing CUI rates is difficult – they can be highly localized or somewhat general in nature. Listed below are some of the environmental conditions that lead to higher CUI rates:
- Marine environments
- Hot or humid environments
- Climates with higher rainfall
- Steam tracing leaks
- Intermittent wet-dry conditions
- Contaminants from the insulation or the atmosphere (such as chlorides and sulfides) dissolving in water
- Systems that operate below typical atmospheric dew point
- Insulation systems that don’t allow moisture drainage
- Insulating materials that hold moisture
CUI is a threat to many industries. If neglected, it remains hidden under the insulation system and becomes only obvious after severe failures.
CUI occurs through penetration of water or moisture and contamination via condensation or external sources (e.g., rain, sprinkler system).CUI can be very localized, with most of the equipment remaining in good condition. This is why sample inspections are unlikely to find all occurrences.
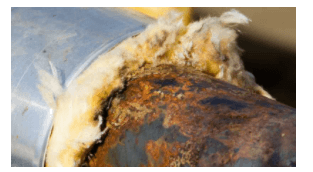 A pipe with corrosion under the insulation.
A pipe with corrosion under the insulation.
Preventing Galvanic Corrosion
Galvanic corrosion results from wet insulation containing a salt electrolyte that allows a current to flow between dissimilar metals. The severity of the attack on the less noble metal depends not only on the difference in potential of the two metals, but also on their relative areas.
Since galvanic corrosion results from water invasion in a humid atmosphere, selecting cellular insulation may be the only answer. It is also recommended to use a plastic or synthetic-rubber jacket that is fire and weather resistant.
The base metals can be also be painted to inhibit cathodic and anodic reactions, and to provide a highly resistive path opposing current flow. However, some paint pigments – such as red oxides and gypsum – can actually promote corrosion, especially in the first coat of primers.
Preventing Alkaline or Acidic Corrosion
Alkaline or acidic corrosion results when an alkali or acid, plus moisture, are present in certain fibrous or granular insulation materials. In a hot service, water vapor may condense at the edge of the insulation and dissolve the alkaline or acidic chemicals there, resulting in corrosion of the aluminum or steel jacketing.
Some alkaline waters with aluminum produce etching and pitting. Pitting can be severe, especially when chloride ions are present. As a safeguard, metal jackets should contain an internal moisture barrier. (Related reading: The Role of Metal Jacketing in CUI.) When corrosion of the jacket is the problem, plastic weather types can be a good solution. In addition, an internally mounted anode beneath the primary weathering barrier and above the secondary coating has been found to be effective as an additional measure.
Preventing Chloride Corrosion
Chloride corrosion is often caused by the combination of insulation containing leachable chlorides with 300 series austenitic stainless steel surfaces at a temperature above 60°C (140°F). Concentration of the chloride ion usually results from the evaporation of rain water or water used to fight fires or of process water. In coastal regions, stress corrosion cracking of insulating jackets is a common issue due to airborne salts. (See Chloride Stress Corrosion Cracking of Austenitic Stainless Steel for more information.)
The probability of failure and the speed of crack propagation are governed by the temperature of the stainless steel and the chloride concentration at the metal's surface. Solutions containing less than 1 ppm are normally considered safe. Below 80°C (176°F), levels of 100 ppm are not particularly dangerous if continuous surface-wetting occurs, but at higher temperatures even lower levels can result in failure.
Water entering the insulation and diffusing inward will eventually reach a region of dry out at the hot pipe or equipment wall. Next to this dry out region is a zone in which the pores of the insulation are filled with a saturated chloride salt solution. When a shutdown occurs and the metal-wall temperature falls, the zone of saturated salt solution moves into the metal wall. Upon reheating, the wall will temporarily be in contact with the saturated chloride solution and stress corrosion cracking may begin.
 Stress corrosion cracking of an insulated stainless steel condensate line. Water wetted the insulation and caused chlorides to leach from
Stress corrosion cracking of an insulated stainless steel condensate line. Water wetted the insulation and caused chlorides to leach from
the insulation onto the hot metal's surface.
Source: NASA Corrosion Engineering Laboratory
To provide protection for the 300 series stainless steel, the insulation must meet MIL-I-24244 or ASTM C-795 specifications. Wrapping equipment with aluminum foil before applying insulation will further reduce the risk of corrosion, because the foil provides a physical barrier that prevents the saturated chloride solution from reaching the metal surface. Due to its high thermal conductivity, the aluminum will be at relatively the same temperature as the equipment, and the chloride solution will shift to the foil, rather than the stainless steel.
Thermal Sprayed Aluminum as a CUI Solution
Thermal sprayed aluminum (TSA) can be used to protect materials against corrosion. TSA is applied by either electric arc or oxy-fuel flame spraying, using solid metal wire. The TSA coating technique is often recommended for carbon steel applications to achieve a life span of over 25 years.
TSA also works well in conditions too severe for organic coatings, such as temperature cycling above and below 149°C (300°F). In the galvanic couple Al-Fe, the steel will be protected cathodically by the aluminum. It acts as a barrier and serves as a sacrificial anode, thus protecting the substrate at the sites of any chips or breaks in the coating.
Detection of Corrosion Under Insulation (CUI)
Knowing where corrosion is likely to occur, such as in low spots and at certain temperatures, helps establish a preventive maintenance program. The National Board Inspection Code 4 and the American Petroleum Institute's API 510 require removal of some insulation at least every five years on all vessels where external corrosion is possible. Maintenance records noting the frequency of trouble spots can assist in setting up an adequate inspection program.
Various Inspection Methodologies for CUI
There are only a few inspection methods to determine the presence of CUI without removing the insulation, and all have certain limitations. Some nondestructive methods include:
Pulsed Eddy Current Testing
Pulsed eddy current testing can be used to detect CUI and can carry out inspection over GI, SS and Al cladding. The insulation can be up to 300 mm thick and the metal thickness can be up to 100 mm. An advanced method, pulsed eddy current array, is very fast. The limitation of this technique is the accuracy of the reading, which can have a variation of 10%, but the most important benefit is that it can be performed while the piping is still in operation.
Long Range Ultrasonic Testing
Long range ultrasonic testing can be done on two-inch diameter pipes or greater. It requires a portion of the Insulation to be removed for the instrument's collar to attach to the pipeline. The ultrasonic waves can detect corrosion in the piping from approximately 5 to 200 meters from the collar, which depends on factors such as the coating, type of corrosion, and whether the pipe is buried. This system can detect corrosion above 3% of the cross sectional area.
Computed Radiography
Computed radiography testing can be performed on the bends of the piping to check for corrosion or erosion. (Related reading: A Look at Digital Radiography for Corrosion Inspection.) This is an accurate system but takes a lot of time to test each bend due to the use of radiography. Also, larger diameter pipes require a cobalt source, thus making use of this technique in a running plant not possible for larger diameters.
Infrared Thermography
Infrared thermography can be of great help for finding moisture under insulation, which in turn may help find CUI.
Recommendations to Prevent Corrosion Under Insulation (CUI)
Corrosion under insulation is an electrochemical corrosion that like all other types of corrosion is manageable. In summary there are two approaches that can be taken to prevent CUI:
- The “one line of defense approach,“ which is essentially the use of insulation on the equipment
- The “two lines of defense approach," which is the application of a coating under the insulation
Having a coating under the insulation is considered the best strategy to control CUI.
One of most frequently used coating options is thermal sprayed aluminum (TSA). The aluminium not only protects the underlying substrate metal but will also act as a sacrificial anode.
The design layout is another factor to be considered. Special care must be taken during the design phase to not inadvertently promote corrosion by permitting water to enter a system either directly or indirectly by capillary action. If the equipment is exposed to an aggressive environment or its geometry creates complications when trying to apply insulation material, then this will definitely increase the possibility of CUI, and the appropriate countermeasures should be taken.