Residual soluble salts can affect coating performance by accelerating the corrosion process and by causing osmotic blistering with subsequent disbondment of the coating. (For background reading, see Five Key Factors in Understanding the Role of Soluble Salts in Coatings Failures and The Role of Soluble Salts in Osmotic Blistering.)
Because of the potential for soluble salts to reduce coating performance, testing the substrate for the presence of these salts should be considered as a requirement of the project specification. Many owners and specifiers make the assumption that soluble salts contaminate all substrates and require the amount of salts on the steel to be reduced to the extent feasible as part of the specified surface preparation process.
There are two widely accepted techniques for testing salt contamination on steel: specific ion detection and conductivity. Specific ion detection will determine whether chlorides, sulfates, nitrates, and ferrous ions are present and in what quantity. Conductivity will not be able to identify the type of salt on the surface; it will only establish the presence of some type of water-soluble salt that is causing the electrical conductivity of water to increase.
Extraction and Analysis of Soluble Salts from Steel Substrates
Salt retrieval methods employed to test for surface concentrations of salt on substrates are automated or manual and fall into three general classes as described in SSPC-Guide 15: Field Methods for Retrieval and Analysis of Soluble Salts on Steel and Other Nonporous Substrates and ISO 8502: Preparation of Steel Substrates Before Application of Paints and Related Products – Tests for Assessment of Surface Cleanliness, parts 2, 5, 6, 9 and 10.
The Class A retrieval method is the most common. It involves automated or manual methods for containing liquid that is held in contact with a surface of a predetermined area. Turbulence within the liquid or a liquid gradient enhances the dissolution of the salt contamination into the solution. The conductivity of a solution is directly related to the total dissolved salt concentration.
All of these devices attach to metal surfaces (magnetically or with non-residue tape). A fixed-volume of pure extraction water is automatically dispensed and agitated against the metal surface to extract or retrieve soluble salts with conductance measurements taken in real-time. The conductance data is digitally processed and stored.
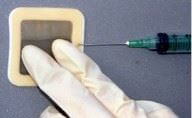
The patch cell retrieval method utilizes a small adhesive patch covered with a latex film, which attaches to the structure forming a cell cavity to the surface being analyzed. Self-contained adhesive edges allow the cell to adhere to the surface. A measured amount of distilled or deionized water or a proprietary extraction liquid is then injected into the cavity with a hypodermic needle. The patch fills up like a large paint blister. The liquid is massaged against the surface being tested, retrieved from the patch using the hypodermic needle and analyzed for concentration of ions.
The sleeve retrieval method uses a small flexible chloride-free latex sleeve (sock) with a self-contained adhesive edge that is attached to the structure being tested, forming a cavity. A salt-retrieval solution is dosed into the sleeve prior to its attachment to the surface. The solution is massaged against the surface being tested for a specified period of time to extract the soluble salts. The sleeve then is removed and the extract is analyzed for levels of soluble salts as required.
The above methods can be used to help characterize surfaces encountered in either laboratory or field settings. No field or laboratory method is believed capable of retrieving 100% of the soluble salts from a surface. The proportion of salt retrieved by field methods depends on the method used, the roughness of the examined surface, the degree of rusting, and the ambient conditions. The presence of pits or deep craters on the surface can lead to grossly inaccurate measurement of salt contamination because salts in the bottoms of deep pits may escape extraction.
Manual or Multi-step Conductivity Analysis
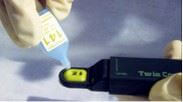
Several instruments are available for measuring conductivity of manually extracted aqueous solutions, including pocket and external cup conductivity meters as well as special field conductivity meters for analyzing filter paper for soluble salts. These units generally compare reagent water against the extracted liquid. (Learn about these and other methods in the article Field Methods for Extraction and Analysis of Soluble Salts.)
Techniques Available to Reduce Salt Levels on Steel Surfaces
A cost-effective approach is to automatically assume that soluble salts are present and incorporate requirements for measurement and reduction of salt levels in the specified surface preparation process. Steam cleaning the surface followed by dry abrasive blast cleaning is a widely used method to reduce salt levels. Other effective surface preparation methods include ultra-high pressure waterjetting, high-pressure waterjetting with abrasive, and wet abrasive blast cleaning.
Once the surface salt reduction process is complete, it is best practice to perform a test to verify that the soluble salts have been reduced to the level specified. Testing can be accomplished by extracting a soluble salt solution from the substrate and obtaining a conductivity value.
Summary
There is no industry consensus standard defining an acceptable soluble salt level. SSPC believes that residual soluble salts can cause premature coating performance failures and recommends that owners should specify reduction of soluble salts prior to protective coating application on steel surfaces to levels based on studies performed to date.
To learn more about the effect of soluble salts on coatings performance and other aspects of good painting practice, visit sspc.org or phone 877.281.7772 to order the complete SSPC Vol. 1: Good Painting Practice.