Metals play an essential role in everyday life. From jewelry to building and bridge construction, metals are considered to be one of the most versatile construction materials available. However, most people are unaware that metals are available in two general classifications: ferrous and non-ferrous.
These two types of materials possess different compositions and properties that lend themselves to different applications.
In this article, we will outline the characteristics that distinguish ferrous from non-ferrous metals and describe how they are used in various applications.
The word ferrous derives from the Latin word "ferrum," which means iron. Ferrous metals are, therefore, those that consist mainly of iron (Fe). These metals may also contain one or more other alloying elements. The use of ferrous metals can be traced back to 1,200 BC, where the development of iron production ushered in the Iron Age.
The presence of iron gives these materials unique properties that are especially valuable in the construction industry. One of the most defining characteristics of ferrous metals is their superior tensile strength and ductility. (Learn more about these characteristics in An In-Depth Look at Tensile Strength.)
Carbon steel, for example, is one of the most popular ferrous metals and is a staple in the construction industry. The properties of this material make it ideal for constructing various structures such as buildings and bridges. Other ferrous metals are used in demanding applications where strength and durability are paramount, such as shipping containers, industrial piping, railroad tracks and automobiles.
While ferrous metals are renowned for their strength and ductility, the presence of iron makes them vulnerable to rusting when exposed to air and moisture. For this reason, some ferrous metals are alloyed with other elements to increase their corrosion resistance. Stainless steel, for example, is a ferrous alloy that exhibits resistance to corrosion due to the presence of chromium. (Discover more in the article Why is Stainless Steel Corrosion Resistant?)
In addition to carbon steel and stainless steel, this metal classification also consists of wrought iron, cast iron and alloy steel.
Non-ferrous metals, as their name implies, are metals that do not contain iron (ferrite). These types of materials have been in use since the Copper Age (around 5,000 BC), where copper was first used to make pottery and jewelry.
Modern uses of non-ferrous metals range from roofing and guttering to pipes and electrical components. Because these metals do not contain iron, they usually exhibit better corrosion resistance than their ferrous counterparts.
The lack of iron also gives them characteristics that make them suitable for applications where magnetic properties may be undesirable. Additionally, while non-ferrous metals do not possess the tensile strengths of ferrous metals, they are renowned for their ability to be easily shaped into various shapes, a property known as malleability.
Non-ferrous metals are generally more costly due to their scarcity. As a result, there is a booming industry for recycling scrap components to extract non-ferrous metals. Copper, for example, is the third most recycled metal in the world.
Some commonly used non-ferrous metals include aluminum, copper, zinc, lead, nickel and titanium. Precious and rare metals, such as gold, silver, platinum, mercury and tungsten are also considered to be non-ferrous.
While some of the critical differences between ferrous and non-ferrous metals were touched on earlier, here we will dive deeper into the properties that distinguish these two metals.
1. Magnetism
Iron is a naturally magnetic element, and therefore materials composed of iron will inherit its magnetic characteristics. Consequently, ferrous metals are magnetic, while non-ferrous metals are not. This property allows these two categories of metals to be easily identified and sorted.
Iron’s magnetism is mainly due to its polar molecular construction. The electrons in its atomic ring are arranged in a non-symmetrical fashion. Therefore, when iron is in proximity to a magnetic field, the electrons are easily pulled to one side of the atom.
This property is mainly responsible for the attraction between ferrous metals and magnets. Non-ferrous metals, on the other hand, can only be attracted to a magnetic field if an electric field runs through them, causing their electrons to become polarized.
Explained another way, the electrons in an atom tend to act like tiny magnets (north and south pole). In non-ferrous metals, the electrons align themselves in pairs, whose “tiny magnets” point in opposite directions, causing them to cancel out each other’s magnetic properties. In iron atoms, the magnetic poles of the electrons point in the same direction, giving them a net magnetic effect.
Because iron has intrinsic magnetic properties on a molecular level, its electrons can be easily aligned in the direction of the flow of a magnetic field. This, in turn, creates an attraction between the iron and the magnetic field.
The magnetic property of ferrous metals makes them ideal for motor and electrical applications.
2. Oxidation
Oxidation is the process where an atom loses electrons. Due to iron’s polarity, it is likely to lose electrons to other polar molecules, such as water. This occurs at the anode, where iron reacts with water and oxygen to form hydrated iron (III) oxide, commonly known as rust.
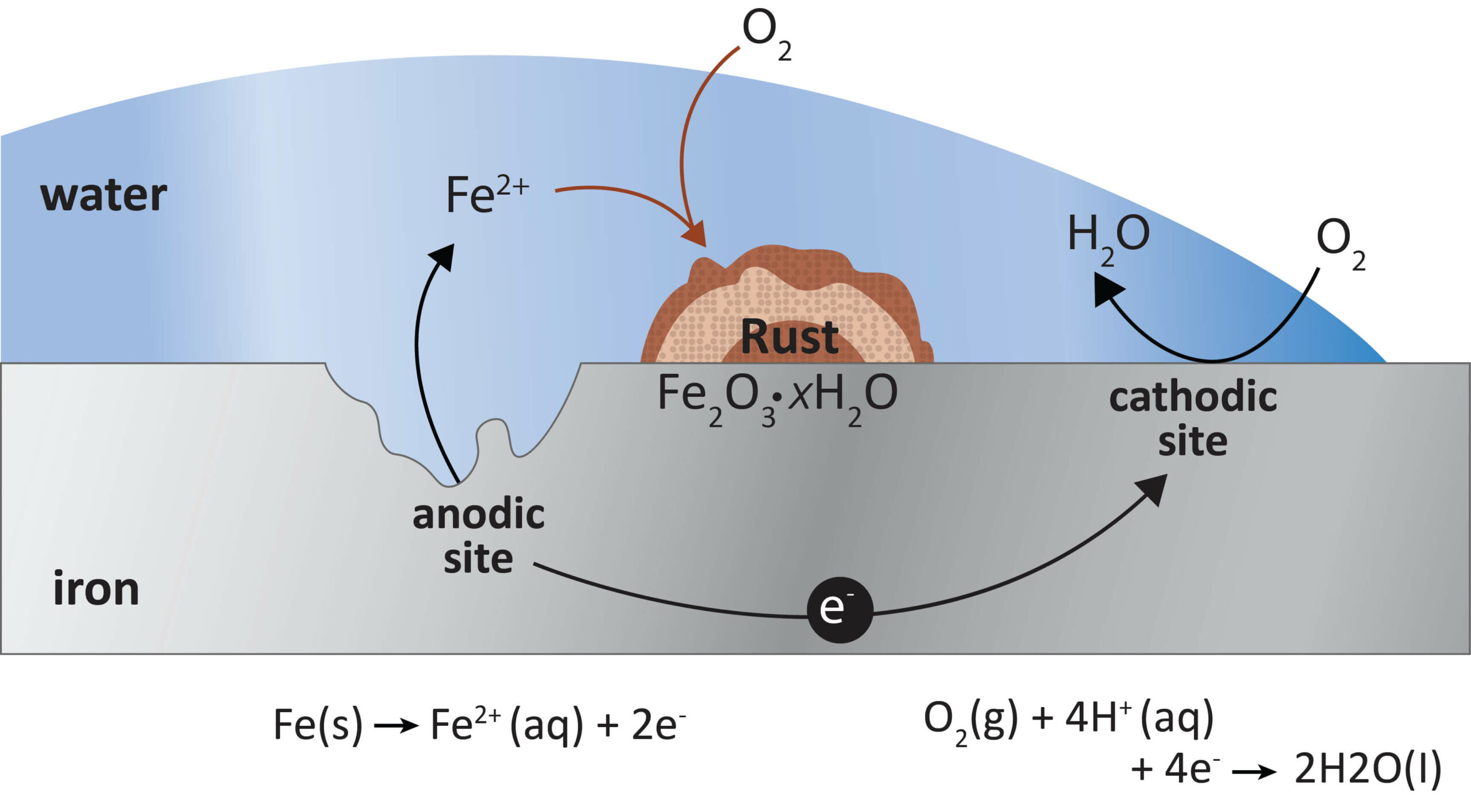
Figure 1. Ferrous surface reacting with air and moisture to cause rusting and degradation.
Since non-ferrous metals do not contain iron, they do not rust in the same way as their ferrous counterparts. Non-ferrous metals such as copper, zinc and titanium react with water and oxygen to form oxide layers that adhere firmly to the surface of the metal and act as impervious barriers. (Learn how this process works in How Metallic Coatings Protect Metals from Corrosion.)
Unlike hydrated iron (III) oxide, which is weak and flaky, zinc oxide and copper oxide are durable and protect the underlying substrate from further corrosive processes. The corrosion-resistant nature of non-ferrous metals makes them ideal for use in highly corrosive environments. Copper pipes, for example, are commonly used to transport corrosive chemicals and sewage.
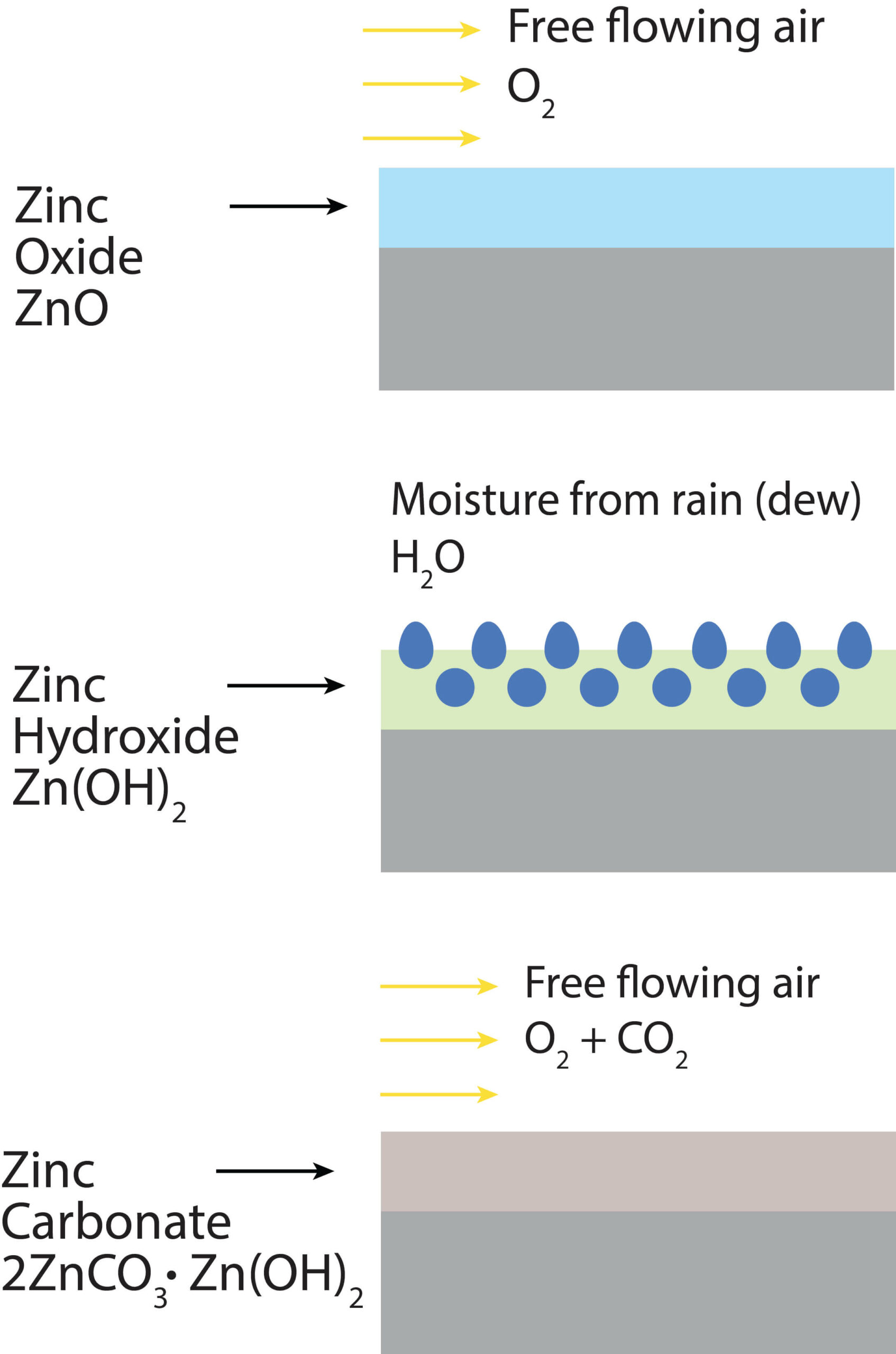
Figure 2. Zinc surface reacting with air and moisture to create a protective zinc carbonate layer.
It is important to note, however, that not all ferrous metals oxidize to develop rust. Stainless steel, for example, is alloyed with chromium, which allows it to generate a stable, protective chromium oxide layer.
3. Tensile Strength
Another feature that distinguishes ferrous from non-ferrous metals is their tensile strength. Ferrous metals, due to the presence of iron, usually have capacities that far surpass non-ferrous metals. Steel, for example, is considered to be one of the strongest construction materials. In addition to high tensile strength, steel possesses a high level of ductility, i.e., it is able to undergo significant deflections without undergoing permanent deformation.
While there are also some high-strength non-ferrous metals, few of these materials are capable of resisting the same forces as iron alloys. Additionally, while titanium has strength properties that are on par with steel, it is also significantly more expensive.
Therefore, titanium is not typically used as a building material. (Learn about the effects of corrosion on this non-ferrous metal in 5 Things to Know and Understand About Titanium Corrosion.)
| Element |
Young's Modulus
(GPa) |
Offset or Yield Strength
(MPa) |
Ultimate Strength
(MPa) |
| Lead |
16 |
|
12 |
| Tin |
47 |
9-14 |
15-200 |
| Aluminum |
70 |
15-20 |
40-50 |
| Gold |
79 |
|
100 |
| Silver |
83 |
|
170 |
| Zinc (wrought) |
105 |
|
110-200 |
| Silicon |
107 |
|
5000-9000 |
| Titanium |
120 |
100-225 |
240-370 |
| Copper |
130 |
117 |
210 |
| Nickel |
170 |
14-35 |
140-195 |
| Tantalum |
186 |
180 |
200 |
| Iron |
211 |
80-100 |
350 |
| Tungsten |
411 |
550 |
550-620 |
Table 1. Tensile strengths of various metals. Notice how the majority of non-ferrous metals possess tensile strengths that are inferior to iron.
What We've Learned
Understanding the differences between ferrous and non-ferrous metals is essential for ensuring that the most appropriate material is used in the intended application.
While the presence of iron distinguishes these two types of metals, this seemingly minor variation gives ferrous and non-ferrous metals very distinctive properties and characteristics.