The galvanic series plays a vital role in determining and preventing corrosion. Also known as the "electropotential series," this series identifies semi-metal and metal nobility. Essentially, galvanic corrosion occurs when two different metals immersed in an electrolyte are joined together. In this scenario, the base or the metal with lesser nobility will undergo corrosion. Thus, the corrosion rate can be determined based on the nobility of metals and the electrolyte to which they're exposed.
Different materials may react with one another through the existence of a catalyst or an electrolyte. In almost all cases, the reaction is not that significant. However, when incorrect materials are combined and exposed to water and other types of electrolytes, the effects can be very harmful. In applications with low humidity, galvanic corrosion is not really a huge problem. On the other hand, in applications subjected to moist or damp conditions, galvanic corrosion may pose a serious threat. (Learn more in the article Top 10 Corrosion Threats.)
The Galvanic Series and Corrosion: A Guide to Material Selection
The relationships in the galvanic series can be a very beneficial guide for choosing metals to join together to avoid corrosion. With the series, material selection can be done effectively so that metals with the least tendency to undergo a galvanic reaction can be chosen. In cases where galvanic interaction is most likely, there will be a need to have a certain level of protection in order to reduce possible potential reactions.
Generally, metals that are located further apart within the series are most likely to undergo galvanic corrosion, which should be stopped through proper selection and design. Metals that are further from each other have the highest rate of corrosion when combined. By knowing the relationships of the metals in the series, galvanic compatibility can be determined, preventing the possible harmful effects of galvanic corrosion.
Chart 1. Galvanic series / galvanic table.
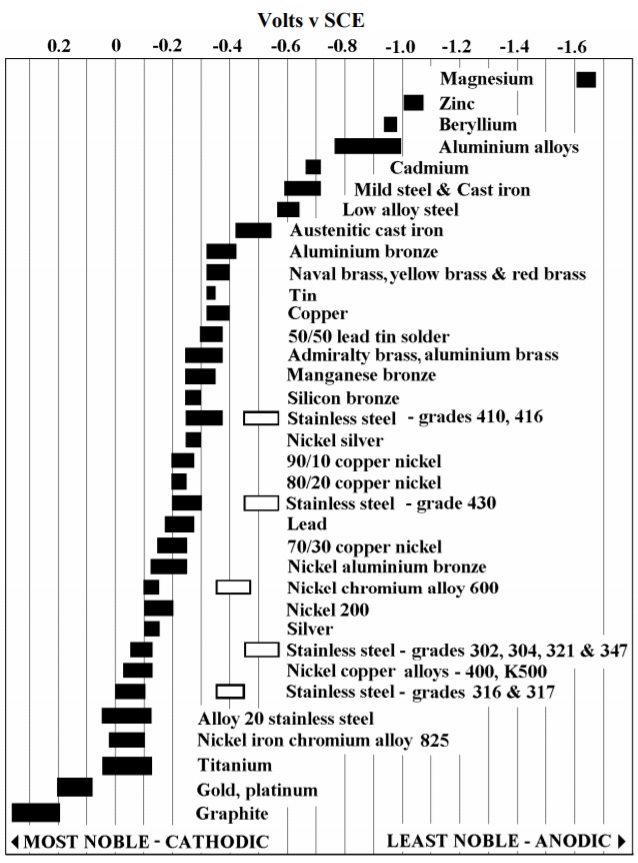
Chart 1. Galvanic series / galvanic table. (Source: Tugsataydin, CC BY-SA 4.0, via Wikimedia Commons)
The chart above shows corrosion potentials in flowing seawater at ambient temperature. The unshaded symbols show ranges exhibited by stainless steels in acidic water such as may exist in crevices or in stagnant, low velocity or poorly aerated water. The more noble materials on the left side tend to be cathodic and hence protected; those on the right are less noble and tend to be anodic. They will therefore be the ones to corrode in a galvanic couple.
The Galvanic Corrosion Table Explained
In order to gain a better understanding of the galvanic series, it is very important to become familiar with the galvanic table. What is a galvanic table?
Basically, this table lists active metals according to the order of the relative activity within an electrolyte environment. The table starts with the most active, or anodic, metal in the series and lists metals down to the cathodic, or least active, metal. The list starts with the most active metals that are most likely to undergo corrosion, such as magnesium, alloys of magnesium, aluminum and zinc. The metals that are last on the list are considered cathodic and the least likely to undergo corrosion.
The galvanic series is applied to a certain solution. In a couple, metals that are on top of the list are considered as the anodes, and will undergo corrosion in preference to the environment. The key to avoid possible damaging corrosion reactions brought about by the combination of metals is to make use of materials that are near each other in the galvanic table. By doing so, galvanic corrosion can be prevented. To achieve the perfect mix of materials, galvanic compatibility should be examined very carefully.
Understanding Galvanic Compatibility
There are times when a particular design requires a combination of different metals. In such cases, the galvanic compatibility is managed through plating and finishes. The chosen methods for plating and finishing facilitate the contact of dissimilar metals, while providing protection to the base metal against corrosion. (Discover more about plating see How Metallic Coatings Protect Metals from Corrosion.)
For example, harsh settings like salt environments, high humidity areas and the outdoors require not higher than 0.15 volts based on the anodic index. For instance, metals such as nickel and silver produce a 0.15 volt difference, which is fairly acceptable. Moving forward, in standard environments like warehouses, storage and other environments with controlled temperature and humidity, the required anodic index difference between metals should not be more than 0.25 volts, while in settings where humidity and temperature can be tightly controlled, a difference of 0.50 volts can be tolerated. But then, extreme caution should be observed for various applications since temperature, humidity and other factors vary in every area. (See the article Temporary Corrosion Protection during Storage, Transportation and Handling for an in depth discussion.)
To prevent the occurrence of galvanic corrosion in various settings where metals and materials are joined, galvanic compatibility should be taken into account through the determination of the acceptable anodic index difference of materials.
By following galvanic compatibility, two metals that do not adhere to the required anodic index can be adequately protected. Metals that are listed further apart from each other in the galvanic table should be protected if these will be used together. Certain measures should be applied to prevent two different metals from coming into contact. This kind of protection can be achieved through various means such as:
- Sealing: This method covers all watertight areas.
- Sacrificial anode: The application of a sacrificial coating to the cathodic element that has a similar potential to the anodic area will prevent the incidence of galvanic corrosion.
- Resistance: Developing resistance through coating, plating and other methods can help increase protection against electrical circuits.
The rule in order to avoid galvanic corrosion is to keep anodic areas away from comparatively cathodic regions.
Galvanic Corrosion Explained
As discussed earlier, galvanic corrosion takes place when two different metals come into contact within an electrolyte capable of conduction, like ground or rainwater. In this type of corrosion, atoms of metal oxidize and leave behind the bulk metal once one or several electrons undergo exchange or transfer. The area where the atoms of metal drop electrons is referred to as the anode; the area where the electrons are transported is the cathode.

Figure 1. Galvanic corrosion due to differing anodic index between the bolts and the plate. (Source D3j4vu at English Wikipedia, CC BY-SA 3.0, via Wikimedia Commons)
The galvanic series lists metals according to their electrical potential in an electrolyte such as seawater. As mentioned, metals that are anodic will corrode at a more rapid rate than passive or cathodic metals.
It is extremely important that the galvanic corrosion potential is considered when it comes to selecting metals for various applications such as trim, paneling, fasteners and more. (The topic of applying coatings to fasteners is discussed in detail in the article High Pressure Fastener Coating Practices Under Fire: Ian MacMoy Speaks Out.)

Figure 2. A variety of metal parts with differing galvanic compatibility.
To minimize galvanic corrosion, it is vital to use similar metals. In circumstances where this is not possible, ample protection should be provided to prevent metals that are not the same from forming an electric connection with water or an electrolyte. (A specific example of marine corrosion is discussed in How to Stop a Ship’s Marine Corrosion in 3 Steps.) Also, keeping tiny anodes from coming into contact with big cathodes can help in reducing the potential for galvanic corrosion.
The corrosion rate relies on the anode relative to the surface of the cathode. If the cathode area is larger than the anode area, there is a higher electron flow of concentration; the result will be galvanic corrosion at a faster rate. When taken the other way around, such as when the cathode area is smaller in comparison to the anode surface, corrosion will occur at a slower pace.
Sacrificial Coatings to Prevent Galvanic Corrosion
In order to achieve galvanic protection, a sacrificial coating is usually used. This gives the passive metal or the base an effective coating that is more anodic compared to the base. The ability of the coating to provide protection is associated with the coating's thickness.
Non-sacrificial and other types of coatings such as the typical paint or plastic coating can also yield protection against galvanic corrosion. However, there are cases when even the smallest scratch on the surface could be the main cause of rapid corrosion, especially when the base metal acts as the anode in the reaction close to a different metal on a huge surface area.
The Galvanic Series in a Nutshell
With the galvanic series, metals are grouped according to electrical potential compared to a known standard. Metals that are far apart from each other on the list will corrode fast in seawater or electrolytes when electrically connected. Metals that are closer together will suffer less damage.