What Does
Galvanic Cathodic Protection (Galvanic CP) Mean?
Galvanic cathodic protection is an corrosion prevention method that uses electrochemical means to protect a base material from corrosion. It does this through the use of a sacrificial anode that corrodes before the material being protected by the sacrificial anode. Galvanic cathodic protection is one of the most commonly employed forms of cathodic protection because of its ease of use.
Corrosionpedia Explains Galvanic Cathodic Protection (Galvanic CP)
Galvanic cathodic protection requires a sacrificial anode that is more electrochemically reactive than the material to be protected. Since the sacrificial anode is more electrochemically reactive, it will corrode before the protected material, so long as they are electrically connected. Sacrificial anodes are available in many different shapes and sizes. An example of a sacrificial anode is a block of zinc that is attached to a steel plate.
Galvanic cathodic protection relies on the potential difference between the sacrificial anode and the cathode, or the material being protected from corrosion. The larger the potential is, the more protection there will be. Galvanic corrosion protection is simple because it does not rely on external electrical sources. However, when the potential difference from the two materials alone is not sufficient to protect the cathode then the potential must be increased through the use of specialized equipment. This is a different type of cathodic protection called impressed current cathodic protection.
Cathodic protection is frequently used to protect:
- Pipelines
- Maritime vessels
- Oil platforms
- Storage tanks
- Underground infrastructure
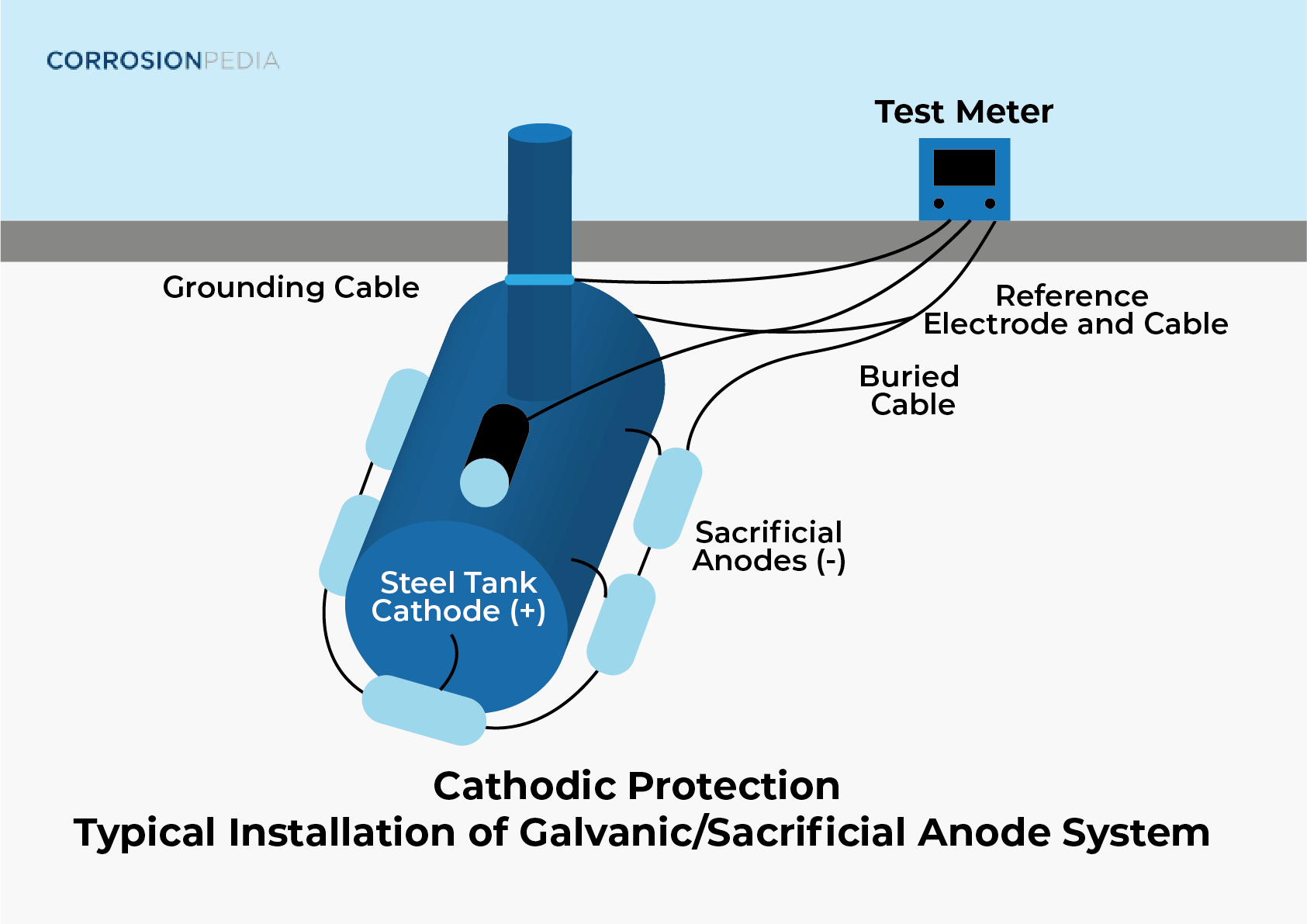
Figure 1. Typical galvanic sacrificial anode system.