Aluminum is the second most abundant metallic element on earth. It has been estimated that 8% of the earth's crust is composed of aluminum, usually found in the bauxite form. On a volume basis, aluminum has become the most widely used non-ferrous metal.
Primary aluminum production is the quantity of primary aluminum produced in a specified period and the quantity of molten or liquid metal tapped from pots that is weighed before transfer to a holding furnace or before further processing.
According to the International Aluminium Institute, 5,414 thousand metric tons of aluminum was produced globally in March, 2019.
The Chemical Nature of Aluminum and Aluminum Corrosion
Aluminum is an amphoteric metal and can react with both chemically acidic and basic substances. When freshly produced, aluminum is highly reactive and reacts spontaneously with water and/or air, instantly forming a thin layer of aluminum oxide (alumina) on its surface. This is one of the reasons why aluminum anodes are used as a galvanic anode for cathodic protection (CP) systems.
The good performance of aluminum in corrosive environments is due to the passivity produced by this protective oxide film. The protective film is an electrical insulator but has a relatively high thermal conductivity (30 Wm-1K-1).
Oxygen influences the corrosion of aluminum and accelerates the corrosion process. The attack increases in high concentrations of dissolved oxygen, especially in acidic solutions. In the case of deaerated solutions, the corrosion rate is very slow. (Learn more about calculating corrosion rates in the article Corrosion Rate Conversion: Simple Ways to Convert Data Between Common Corrosion Units.)
The chemical formula for the reaction of aluminum in the presence of oxygen is:
4Al + 3O2 –> 2Al2O3
The following are possible reactions of aluminum with water:
2Al + 6H2O –> 2Al(OH)3 + 3H2
2Al + 4H2O –> 2AlO(OH) + 3H2
2Al + 3H2O –> 2Al2O3 + 3H2
All of these reactions are thermodynamically favorable from room temperature through the melting point of aluminum at 660°C (1,220°F). All are also highly exothermic. From room temperature to 280°C (536°F), Al(OH)3 is the most stable product, while from 280-480°C (526-896°F), AlO(OH) is most stable. Above 480°C (896°F), Al2O3 (alumina or aluminum oxide) is the most stable product.
In normal atmospheric conditions the oxide film is composed of two layers (Figure 1). The inner oxide layer closer to the metal base is a compact amorphous barrier layer of a thickness determined only by the temperature of the environment; the limiting barrier thickness is the same in oxygen, dry air or moist air. Covering the barrier layer is a thicker, more permeable outer layer of hydrated oxide.
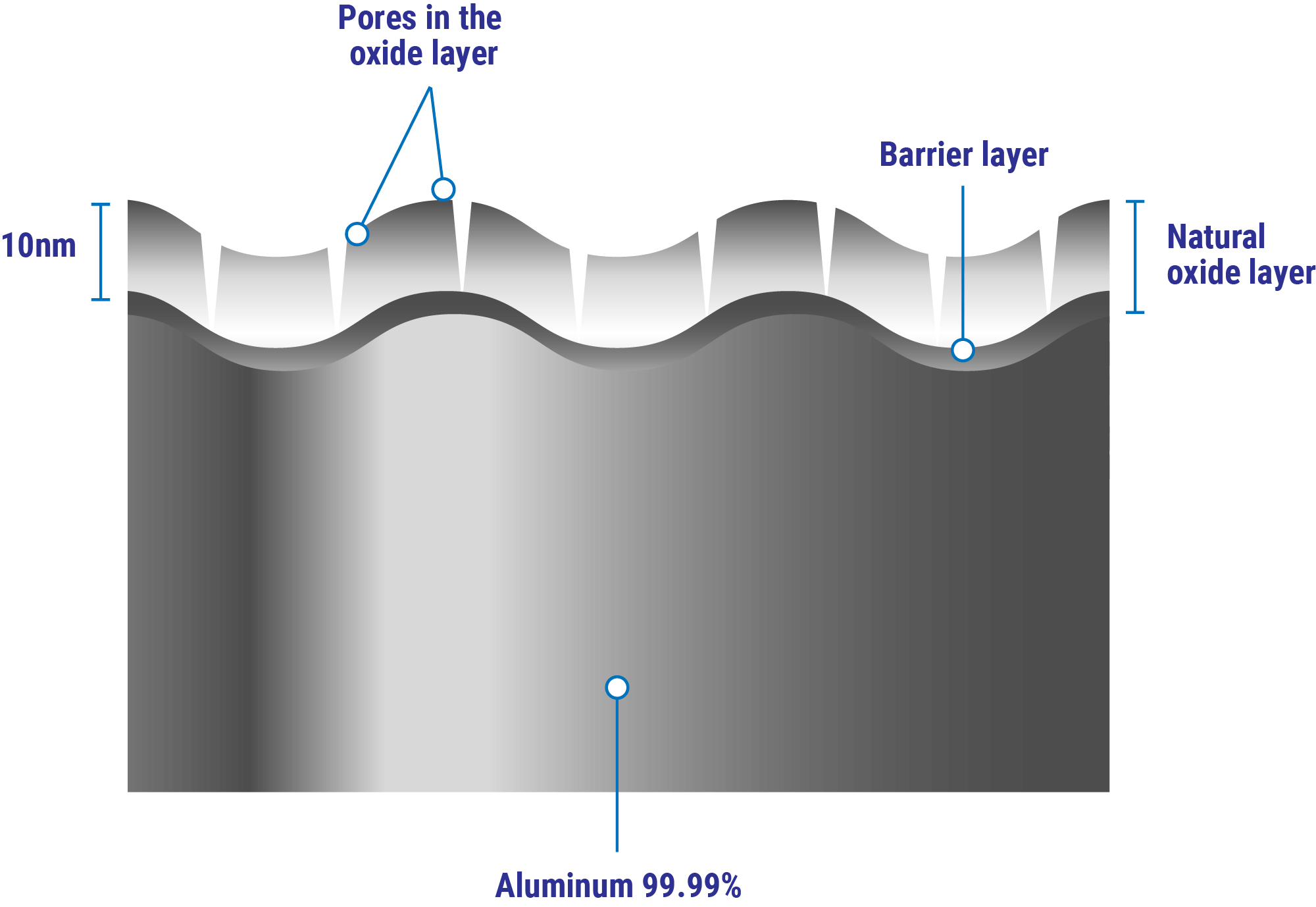
Figure 1. Schematic of the passive oxide film that forms on aluminum.
The conditions for thermodynamic stability of the oxide film are expressed by the Pourbaix diagram (Figure 2). As the diagram shows, aluminum is passive only in the pH range of approximately 4 to 9; the limits depending on the temperature. At higher temperatures the oxide film may consist of a thin amorphous barrier layer next to the aluminum and a thicker crystalline layer (boehmite, aluminum oxide hydroxide AlOOH) next to the barrier layer. At lower temperatures, the predominant forms produced by corrosion are bayerite and aluminum oxide trihydroxide Al(OH)3. Because the form of aluminum oxide produced depends on corrosion conditions, its identification is sometime useful in establishing the cause of corrosion.
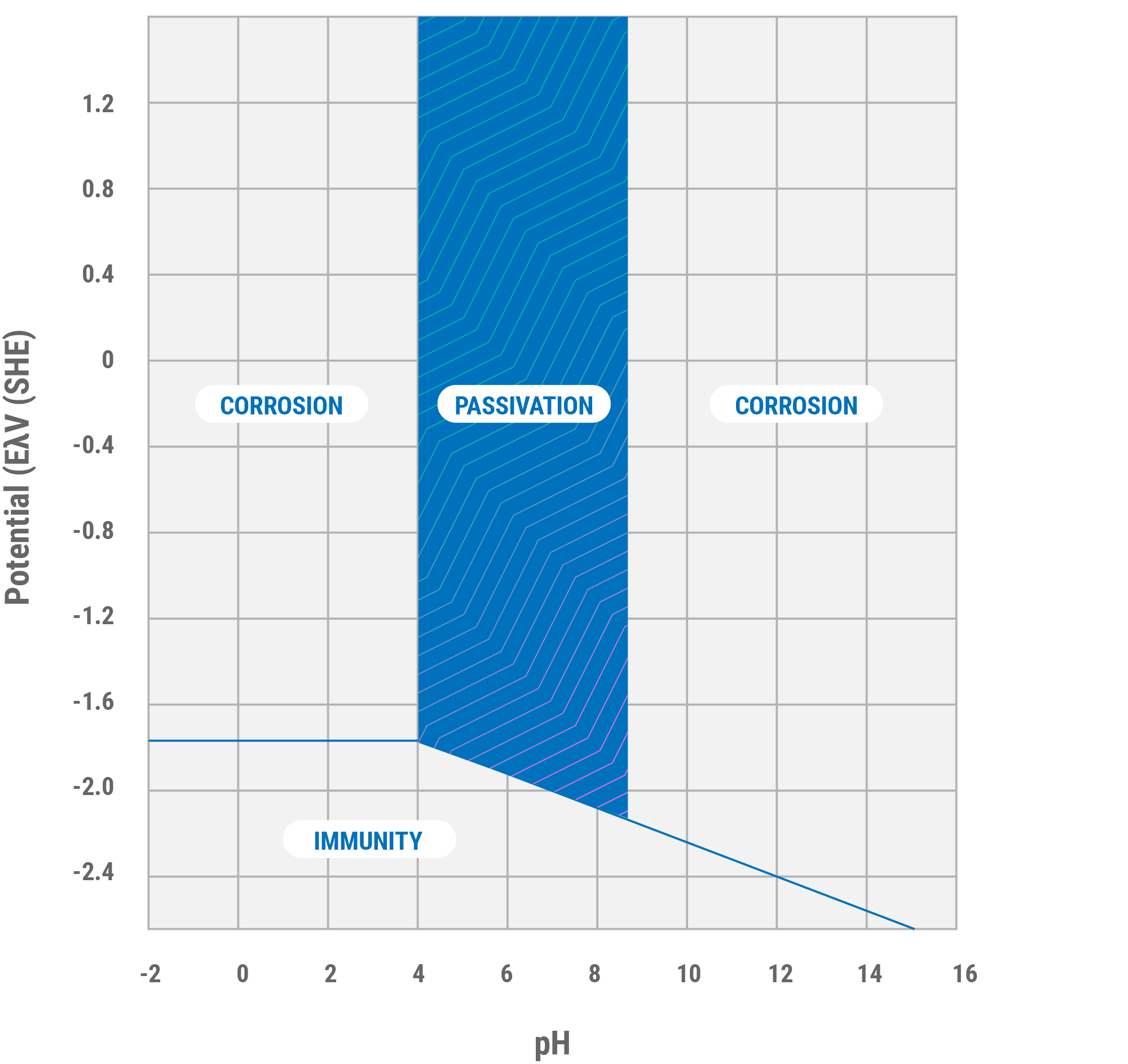
Figure 2. Pourbaix diagram for aluminum showing the regions of corrosion, immunity and passivation of aluminum at 25°C.
Beyond the limits of its passive range (pH range of 4 to 9), aluminum corrodes in aqueous solutions because its oxides are soluble in many acids and bases. When this layer comes in contact with chlorine it produces a reaction that releases hydrogen gas, which creates dialuminum hexachloride (AlCl3). In aqueous conditions it forms an acid mist that breaks down the protective layer, with the final result being various forms of corrosion such as crevice corrosion, intergranular corrosion, galvanic corrosion, etc.
If this oxide thin film is damaged it can be reformed (re-rust) immediately in most environments and continue to protect the aluminum from corrosion, but when the film is removed or damaged under conditions such that self-repair cannot occur, then corrosion occurs. (Discover self-healing metal oxides in A Look at Self-Healing Metal Oxides as a Corrosion Prevention Method.)
The growth of oxide films on metals at ambient pressures and low temperatures has been addressed in detail by Cabrera and Mott. In their model, electrons from the metal traverse the growing oxide, forming anions with the oxygen on the surface. The negative oxygen anions and the positive metallic cations below the oxide create a so-called Mott potential.
The Mott potential decreases the barrier for migration of oxygen anions and/or metallic cations through the oxide. Consequently, the growth rate increases and is extremely rapid initially. Nevertheless, with increasing thickness of the oxide, the additional effect of the Mott potential decreases, and when the effect is too small to significantly affect the ion transport, the oxide growth stops and a limiting thickness is reached.
Some researchers have demonstrated that the aluminum oxide layer could reach about 4 nm of thickness on aluminum metal formed in air at ambient temperature. Considering the environmental conditions, the aluminum oxide layer will show different grows rates.
Applications and Uses for Aluminum Oxide
The major uses of specialty aluminum oxides are in refractories, ceramics, and polishing and abrasive applications. Large tonnages are also used in the manufacture of zeolites, coating titanium pigments and as a fire retardant/smoke suppressant.
Applications and Uses for Aluminum and Its Alloys
Most applications for aluminum utilize alloys having one or more elemental additions, which give improvements in the mechanical, physical and chemical properties of the resulting material. The major alloying additions used with aluminum are copper, manganese, silicon, magnesium and zinc. The total amount of these elements can constitute up to 10% of the alloy composition (% weight). Aluminum and its alloys are nonmagnetic and have high electrical conductivity, high thermal conductivity, high reflectivity and noncatalytic action.
Aluminum Alloy Corrosion
The common aluminum alloys that do not contain copper as a major alloying constituent are resistant to unpolluted seawater. Among wrought alloys, those of the 5XXX series (aluminum alloys in which magnesium is the principal alloying element) have the highest resistance to seawater, and considering their other desirable characteristics, they are the most widely used for marine applications.
Aluminum alloy corrosion in seawater is mainly of the pitting type, as would be expected from its salinity and enough dissolved oxygen as a cathodic reactant to polarize the alloys to their pitting potentials.
The aluminum-base alloys as a class are highly resistant to normal outdoor exposure conditions. The alloys containing copper as a major alloying constituent (over ~1%) are somewhat less resistant than the other aluminum-base alloys, whereas the Alclad alloys are generally the most resistant.
The corrosion attack on aluminum alloys in soil depends on the composition and climatic conditions of the soil. In dry, sandy soil, corrosion is negligible, but in wet, acidic or alkaline soils, the attack may be severe.
Most gases, in the absence of water and at or near room temperature, have little or no action on aluminum-base alloys. In the presence of water, the acid gases, such as HCl and HF are corrosive, and wet SO2 causes corrosion. Hydrogen sulfide, ammonia and halogenated hydrocarbons in either the presence or absence of water and at room temperature or slightly above, has negligible action on aluminum-base alloys. However, methyl chloride and methyl bromide are corrosive and should not be used in contact with aluminum-base alloys.
Phenols and carbon tetrachloride nearly dry or near their boiling points are very corrosive to aluminum alloys. This behavior can be prevented by the presence of trace water.
The action of metallic mercury on aluminum is unique. It tends to amalgamate readily with aluminum at room temperature to produce an extraordinary corrosion rate in the presence of moisture with the production of voluminous columnar corrosion products.
Electrical Current Flow as a Cause of Aluminum Corrosion
The causes and forms of aluminum corrosion are associated with the flow of electric current between anodic and cathodicregions. The intensity of corrosion in this case depends on the difference of potential of the two regions, which is due to microstructural defects caused by fabrication, welding and other joining methods. The effect is further augmented by the difference in the electrical potential of the alloying materials (the alloy is never perfectly homogeneous, so there are microregions where the alloying material can be found in slightly larger quantities). (For more details, see The Corrosion Properties of Aluminum and Its Alloys.)
The forms of corrosion on aluminum and its alloys can be any of the following types: