A welded joint can have a low resistance to corrosion due to the varying chemical composition, residual stress and metallurgical structure of the weld zone. Corrosion of weld joints can be avoided by the careful selection of materials to be welded, the filler metal, welding techniques and finishing. However, even after closely matching the metals and using the best techniques, weld corrosion may still occur due to a variety of reasons. (Discover more in An Overview of Welded Joint Corrosion: Causes and Prevention Practices.)
Factors that Lead to Corrosion in Welds
The Venn diagram below (Figure 1) shows how the material, the environment and stresses contribute to different types of corrosion in welds.
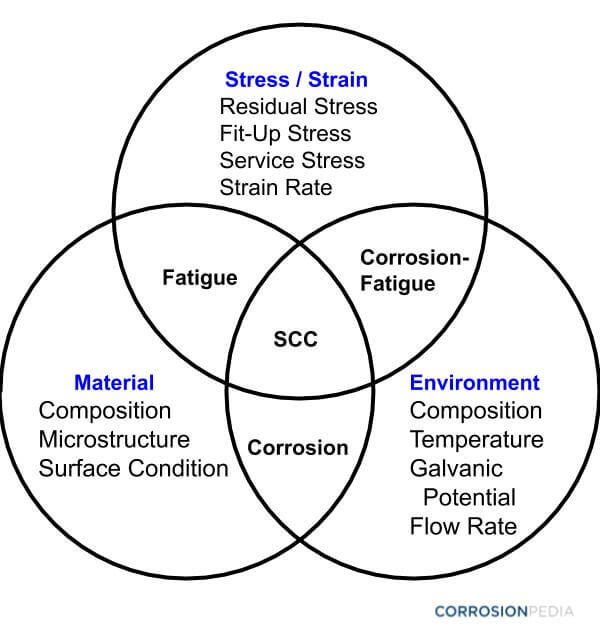
Figure 1. Interrelationship between stresses, materials and environmental factors that lead to weld corrosion.
The metallurgical, physical and chemical changes caused by the welding process affect the corrosion resistance of the weld. This leads to both the heat affected zone (HAZ) and the weld metal corroding faster or slower than the base metal. Cases of uniform corrosion across the base metal and weld metal, or the base metal corroding and the weld metal remaining unaffected, are also possible.

Figure 2. An example of corrosion around a weldment.
The heating and cooling cycles arising from the welding process usually affect the surface and microstructure composition of the weld deposit and the adjacent base metal. This may reduce the corrosion resistance of both the base metal as well as the weld material.
Some of the factors that reduce corrosion resistance are contamination of the solidifying pool, recrystallization and grain growth in the weld HAZ, formation of unmixed zones, precipitation of secondary phases and microsegregation.
However, corrosion resistance can still be maintained by balancing the alloy composition to prevent precipitation, shielding the hot and molten metal surfaces from reactive gases from the weld environment, choosing the appropriate welding parameters, removing chromium-depleted base metal, and removing the chromium-enriched oxides from the heat-tinted surfaces.
Types and Causes of Corrosion on Welds
Welds experience all types of corrosion; however, they are more susceptible to the forms arising from variations in composition and microstructure. Specific corrosion types are galvanic, stress corrosion, hydrogen cracking, intergranular and pitting corrosion.
- Variations in the composition of the base metal, HAZ and weld metal result in a condition that favors galvanic corrosion.
- Susceptibility to hydrogen-containing environments will often lead to cracking.
- Welding residual stresses lead to stress corrosion cracking (SCC).
- The presence of hydrogen in the welding process leads to hydrogen-induced cracking of the weldments. Hydrogen can arise from improperly baked or poorly stored electrodes, the presence of impurities and moisture in the components to be welded, or the presence of moisture in the flux.
- Weld discontinuities like surface flaws can act as preferential sites that lead to local corrosion attacks.
Galvanic Couples
Different compositions of the base metal and the filler metal may lead to galvanic couples. This gives rise to an electrochemical potential difference and causes some regions in the weld to be more active.
Galvanic corrosion is particularly an issue when the weld joint is used in a harsh environment such as in seawater. (Read Corrosion Fatigue of Welded Joints on Marine Offshore Structures for more information.) It is therefore necessary to carefully select the appropriate filler metal for harsh environments.
Weld Decay of Stainless Steel
During the welding of stainless steels, regions susceptible to corrosion may develop. The process, called sensitization, is caused by the formation of chromium carbide along the grain boundaries. Sensitization depletes the chromium from the regions adjacent to the grain boundary, leading to the formation of localized galvanic cells.
If the chromium content drops below the 12% necessary to maintain a passive film, the region become susceptible to corrosion, and is likely to suffer intergranular attack. This attack results in weld decay and is most common in the HAZ.
Sensitization can be minimized by post-weld high-temperature annealing and quenching. This redissolves the chromium at the grain boundaries and prevents the formation of chromium carbide during the cooling process.
The attacks on HAZ can be minimized by post-weld heat treatment (PWHT) stress relief. However, it is much easier to avoid the effect through appropriate material selection and proper welding procedures such as:
- Using a low-carbon stainless steel such as the 304L and 316L, and hence prevent the formation of carbide.
- Using post-weld heat treatment.
- Using stabilized grades alloyed with niobium or titanium such as the 347 and 321 respectively. Niobium and titanium are strong carbide-formers that react with carbon and prevent the chromium depletion.
Note: See An Introduction to Stainless Steels for more information about various stainless steel grades.
Preferential Weld Corrosion (PWC)
Preferential weld corrosion occurs in welds when exposed to seawater and other corrosive environments. The weld metal compositions are usually optimized to enhance their mechanical properties; this makes them more anodic than the base steel, causing them to corrode at higher rates compared to their base metals.
Reasons for corrosion include:
- The difference between the composition of the base metal and the weld metal may be sufficient enough to form a galvanic cell and corrosion.
- The resulting as-welded microstructure may alter and reduce the weld metal corrosion resistance.
- The differences in the microstructures between the as-welded HAZ and the base metal can lead to the localized attack of the heat-affected zone.
The preferential corrosion of welds is more likely to occur when the material is in contact with a highly electrically conductive media such as seawater. However, it can also occur in CO2 environments of lower conductivity.
The preferential weld metal corrosion is minimized by adding alloying elements to make the weld metal more cathodic than the adjacent parent metal.
Welding Practices to Minimize Corrosion
Optimized material selection and welding procedures can assist in producing a corrosion-resistant weld. Full weld penetration, post-weld dressing and avoidance of excessive weld reinforcement are some of the effective ways of minimizing the weld geometrical effects.
Material Selection
The careful selection and matching of welding materials and consumables will reduce the difference in the micro and macro composition across the weld, thereby reducing galvanic conditions.
Surface Preparation
Contaminant elements and compounds at the surfaces to be welded must be removed, otherwise the heat during the welding process can cause weld defects, cracking and reduce the corrosion resistance of the heat-affected zones or the weld itself.
Sulfur, phosphorous and low-melting elements can lead to cracks in the HAZ or the weld. If the carbon or carbonaceous materials are left on the surface when welding, they may be taken in solution and result in a high carbon layer, which reduces the resistance to corrosion in certain environments.
Care should be taken to ensure that the cleaning process does not introduce other problems. (Learn more in Substrate Surface Preparation for Corrosion Prevention.)
Weld Design
A poor weld design may produce crevices that trap stagnant solutions, eventually leading to pitting or crevice corrosion. The irregular deposit shapes in tabular products will, for example, promote turbulent flow and result in erosion corrosion. The weld deposits in a good design should have relatively flat beads, which have low profiles and minimum slag entrapment. A careful fit-up will also prevent locked-in stresses. (Learn more in the article How to Control Corrosion by Improving Design.)
Welding Process
It is often recommended to make a sound joint through complete penetration. This avoids the underbead gaps. In addition, one should remove the slug after each pass. This can be done using a chipping tool or grinder. The geometry of the weld joint should be designed in a way that allows for the complete removal of the corrosive and hydrophilic flux residues.
Use of Backing Material
This is recommended when welding plates or sheets, and when only one side is to be welded. If backing is not used, the side underneath may have erratic penetration with voids, crevices and excessive oxidation. These defects may initiate corrosion in addition to reducing the weld strength. Copper is the preferred material for the backing bars due to its high thermal conductivity.
Weld Surface Finishing
It is essential to inspect the weld deposit immediately after the welding process. For maximum resistance to corrosion, the surface must be smooth, uniformly oxidized and free of irregularities and other foreign particles. Grinding may be used to even out the roughness and weld spatter, while a wire brush may be used to smooth out the surface. However, brushing is not recommended for stainless steels since it may disturb the passive film and reduce its resistance to corrosion.
Removing the Sources of Hydrogen
Using welding consumables such as low-hydrogen shielded metal arc welding electrodes, cleaning the welding surfaces and drying the flux will reduce the presence of hydrogen pickup and hence prevent the associated hydrogen-induced cracking.
Application of Surface Coating
A protective surface coating applied to the welded joint as well as the parent metal minimizes the chances of localized corrosion, which may arise due to the variations in the weld metal composition.
Post-Weld Heat Treatment (PWHT)
The treatment reduces the residual stress gradients and is an effective method of reducing the susceptibility to stress corrosion cracking. The PWHT reduces the composition gradients and the formation of microgalvanic cells. The other role of the treatment is to transport hydrogen from the weld regions and hence prevent hydrogen cracking.
Conclusion
Corrosion in welds may occur due to several contributing individual factors or a combination of factors. The effects of these factors can be minimized through the appropriate selection of materials and welding procedures. Stress-relieving after welding, slag removal, avoidance of crevices, sharp notches, rough finish and galvanic couples are some of the main preventive measures that can minimize the possibility of corrosion in welds.