Corrosion fatigue is a major concern when the integrity and safety of any offshore structure is concerned. These structures, as well as ships, are subjected to the cyclic stress produced by waves and tidal motion. While the effect of fatigue, caused by cyclic loads, is fairly well understood, the combined damaging effect of fatigue and corrosion can have unexpected consequences and significantly shorten the life of a structure. (Read more about offshore assets in Industry Experts Discuss Subsea Pipeline Corrosion Management.)
What is Corrosion Fatigue?
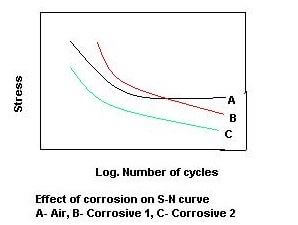
Where cyclic load and fatigue are concerned, the easiest way to visualize the relationship between stress and the number of cycles is to show it on an S-N diagram. If one was to look at such a diagram for a particular specimen where the testing was done in a standard environment, it would be significantly different that the tests done on the same specimen in any kind of corrosive environment. These corrosion fatigue diagrams would be shifted to the left, or have the usual fatigue limit removed, which means that the dynamic properties and fatigue strength of the given material are weakened.
Source: en.wikipedia.org
The synergistic effect of both fatigue and corrosion acting simultaneously makes it difficult to determine the exact interaction between the two, but it is safe to assume that the corrosion fatigue is more than just a simple superposition of the two negative effects. While we can improve the fatigue strength of a material through heat treatment and alloying, if the material is in a corrosive environment the advantage gained can easily be completely neutralized, as the corrosive mechanism in play usually dictates the endurance limit in such a case. In fact, the difference in fatigue strength is most noticeable at lower stress values.
The Observed Effects Compared to Normal Fatigue
The biggest difference between corrosion fatigue and ordinary fatigue is in the crack nucleation and crack propagation time. During the normal fatigue testing of smooth specimens, the crack nucleation period takes up to 90% of the specimen’s lifetime, followed by fairly rapid crack propagation. On the other hand, in the case of corrosion fatigue, the ratio shifts significantly and only around 10% of the specimen life is spent in the crack initiation phase. (Learn how to perform fatigue testing in the article 3 Essential Types of Material Destructive Tests.)
This is the main reason why the endurance limit and the fatigue strength are lower when a specimen is tested in a corrosive environment. Since such a short portion of a specimen’s lifespan is spent in the crack nucleation, it stands to reason that it is more useful to focus on evaluating crack propagation behavior, which means that fracture mechanics is a prime tool used to establish the durability of such a specimen.
To this effect, in order to examine the crack growth behavior, pre-cracked specimens are used and the crack-propagation velocity is measured. Fatigue cracks propagate in a stable fashion, as long as the stress values are below a critical value – the fracture toughness. This sub-critical crack growth can be fairly precisely predicted, giving a good estimate of how long the particular element can operate before the unstable crack propagation, which usually lasts for extremely short periods, leads to an almost instantaneous clean break.
In the case where corrosion fatigue is in play, besides the severely shortened time needed for the initial crack to form, the crack-growth is also faster. The stress threshold is lower and crack-growth velocities are higher for any sub-critical stress value, which means that only a short crack is needed for the element failure.
In this case, two elements play a role in crack growth: load levels and type of corrosion. Common types of corrosion, like pitting corrosion, crevice corrosion, galvanic corrosion and microbial corrosion all affect the crack nucleation and crack growth differently on their own. Combined with the various types of dynamic or cyclic loads that the specimen or construction can be subjected to, corrosion fatigue has a vast range of possible variations, so it is of utmost importance to be extremely familiar with both the load levels and type of corrosion present to determine the safe lifespan of an element that is affected by this mechanism.
How Weld Material Influences Corrosion Fatigue
One of the main reasons why weldments are particularly vulnerable to corrosion fatigue is that welds are usually more susceptible than the base material that the rest of the structure is made of. As previously discussed in An Overview of Welded Joint Corrosion: Causes and Prevention Practices, there are a large number of corrosion mechanisms that plague welded joints, especially in the presence of an electrolyte, such as water.
While welded land pipelines are one of the potential candidates, this particular mechanism is most damaging to offshore structures, ships and underwater pipelines. All these systems are in the presence of very electrolytic saltwater, have a large number of welds and are subjected to cyclic loads caused by the waves, tides and even the standard operation of the construction itself.
There have been several studies and tests undertaken to establish the effects of corrosion fatigue, particularly in the case of stainless steels and carbon-manganese (C-Mn) steels, which are commonly used in offshore risers. These fatigue tests have been conducted in various environments that included saltwater, carbon dioxide (CO2), hydrogen sulfide (H2S) and chloride ions.
Some of the conclusions are:
- Depending on the type of corrosive environment, a specimen would suffer failure two to three times faster than the control specimen would. The fatigue lifespan of a welded joint was reduced by a factor of three at low temperatures (around 5°C / 41°F), but it was more than that at higher temperatures.
- Seawater has a particularly detrimental effect on materials with fine microstructure, such as high-strength low-alloy (HSLA) steels and in recrystallized sections of weld heat affected zones (HAZ), because of the combined effect of fatigue and hydrogen embrittlement on crack growth. The negative effect was present even in materials with cathodic protection (CP).
- Hydrogen sulfide has an extremely adverse effect on fatigue performance, both in dry, oil and water environments. Dry hydrogen sulfide or hydrogen sulfide in oil can increase the crack growth by up to 35 times at high concentrations, with the effect being weaker at lower concentrations. In cases where water contains hydrogen sulfide, the effect is even worse: the fatigue crack growth rate can increase by a hundredfold or more.
How to Prevent or Mitigate Corrosion Fatigue
While there is no real way to completely prevent either corrosion or fatigue, steps can be taken to reduce the effects of these damaging mechanisms and significantly prolong the life of the affected structure.
The most straightforward step is to design a structure in such a way as to minimize, or preferably completely eliminate, cyclic stresses. The correct shape of critical sections and joints can reduce residual stress, help reduce stress concentration and help distribute the load across the whole component. Vibration, flutter and rapid changes of loading, temperature and pressure should be avoided as much as possible.
Cathodic protection might actually be detrimental if hydrogen sulfide is present, so care should be taken to choose the appropriate corrosion protection method. Material should be properly chosen for the particular corrosive environment, and it should be treated in a way that assures the best corrosion resistance for the expected operational temperature, corrosive agent and other factors. It is extremely important to limit at least one of the two factors (fatigue and corrosion), because their combined action has an exponentially worse effect on the reliability and service life of the vulnerable system or structure.