Soluble salts are formed when an ion-bonded anion and cation break apart in the presence of water. Osmotic blistering occurs when the amount of water flowing through the coating to the surface of the steel remains at a constant rate—but due to soluble salts at the surface of the steel, the evaporation rate of water leaving the surface slows down. When the hydraulic pressure exceeds the adhesive or cohesive strength of the coating, a blister forms. This results in what we refer to as osmotic blistering. The liquid is not “pulled” to the surface, as most of the industry literature claims, and blistered paint has nothing to do with how hygroscopic the salt is.
Salts on Bare Steel vs. Coated Steel
The problem with salts on bare steel is completely different to the problem with salts on coated steel. On uncoated steel, all salts are to some degree hygroscopic. That means that above a certain relative humidity (RH), they will draw moisture out of the air and form a solution. Since this solution is conductive, it results in an electrochemical reaction often referred to as flash rust. (Learn more about flash rust in the article 7 Things to Know About Flash Rust.) The relative humidity at which this occurs depends on the species of salt.
The most common salt, sodium chloride, has a RH of 75%, so it is hygroscopic only at a high relative humidity. Other salts, such as potassium chloride, are much more hygroscopic and will react at an RH of 33%.
Since we are discussing a coatings industry problem, let’s focus on soluble salts and coatings. Industry pundits often state that salts are bad because they “draw” water through the coating to the surface. This statement shows a fundamental lack of understanding of the process.
Osmosis is a property of the solution. It is a colligative property, which means it does not matter what type of salt is dissolved, only how much salt is dissolved.
The osmosis process we learned about in school involves a semipermeable membrane with solutions of differing concentrations on each side. Initially, the membrane allows water molecules to pass through but not the substances dissolved in solution. To equalize the osmotic pressure on the membrane, water molecules pass through the membrane from the lower concentration to the higher concentration until the numbers of dissolved particles are the same on both sides of the membrane.
This is not what happens when we are talking about coatings in immersed service. What happens with salts under coatings is not really osmosis because paint is not a semi-permeable membrane. However, the actual process is close enough to osmosis that this models works well enough.
Osmotic Blistering in Immersed Service
To understand what really happens, we need to look at other properties of solutions. When any substance is dissolved in water, it increases the boiling point and slows the evaporation rate.
A simplification helps illustrate this: As the foreign particles dissolve in the H20, some of these particles replace the water molecules on the water’s surface. Since less area of water is exposed to the air, evaporation slows down.
Most coatings in immersion service contain between 1 to 3% water. This water migrates through the coating to the surface of the steel below. If the steel surface is clean and free of contaminating particles, the water continues on its path away from the surface at the same rate it reached the surface, as shown in Figure 1.
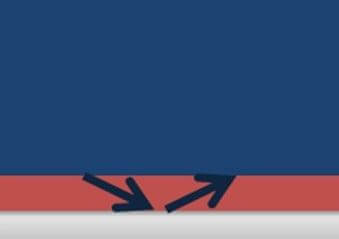
Figure 1. No salts or contaminants present.
However, if the water encounters any soluble compounds when it reaches the surface of the steel, the particles dissolve in the water and this slows down the evaporation rate, as shown in Figure 2.
As a result, the rate of water coming to the steel surface stays the same, but the amount of water leaving the surface slows down. When the amount of water build-up exceeds the adhesive strength of the coating due to hydraulic pressure, a blister forms.
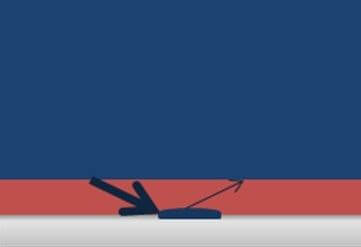
Figure 2. Contaminants slow the rate of evaporation.
All of these principles apply regardless of any particular type of salt. In actuality, the type of salt does not matter, only how much of it is present. This brings us to testing for salts, which the industry standards address as part of proper surface preparation before applying coatings. (This topic is explored more fully in Best Practices for Cost-Effective Surface Salt Removal.)
A big issue of debate in the coating industry is Total Salt testing verses Specific Ion testing. Each has advantages, so which is best? The first clue is that 90% of the world, with the exception of the United States, uses Total Salt testing as described in the standards ISO 8502-6 and ISO 8502-9.
Commonly used Specific Ion tests only test for the anion. But it is often the cation that determines the property of the salt. For example, H+, Na+, K+ and Ca++ are all cations. When combined with Cl-, they all have different properties. If you run a Specific Ion test for the presence of chloride, is the cation hydrogen (HCl), sodium (NaCl) or something else? The Specific Ion test will not tell you.
While a conductivity test will not tell you specifics, the same chloride reading with H+ will give a much higher conductivity reading than the same amount of chloride with Na+.
Testing Ice Cream and Marbles
Here is a commonplace illustration—a method I developed to test for vanilla in ice cream. I walk into my favorite ice cream store that advertises 38 flavors of ice cream. I test all the flavors and find only 13 have vanilla. I inform the manager that he only has 13 flavors of ice cream. I am sure you can see the absurdity of this, but when we run a chloride test, which is the most common salt ion, and find zero chloride ions, how can we assume there are no salts on the surface?
Here’s another illustration using a bucketful of marbles. Let us assume that there are 38 different colors of marbles. I develop a method to test for red, yellow and blue marbles. I find that we have:
- 100 red marbles = chloride
- 50 blue marbles = sulfate
- 25 yellow marbles = nitrite
From this information, how can we calculate the total number of marbles in the bucket? If we test and find that there are no red, blue or yellow marbles, can we then conclude that the bucket is empty? Like the ice cream, just because you test for one or even three ions and do not find them present, it does not mean that other ions are not present.
In the earlier example of osmotic blistering, we have shown that it is the total number of dissolved particles (marbles) that leads to blistering. So, what difference does it make if we know how many red, blue and yellow marbles are present if we don’t know the total number of marbles?
Recommended Salt Test Methods
Total Salt testing uses conductivity to determine the total number of marbles, but this test will not tell you what color the marbles are. The ISO standard makes it easy for users by assuming that all salts found should be reported as sodium chloride. Unfortunately, I believe this confuses the issue. I still prefer to report the number as total salts.
Another test that works well to determine the presence of salts uses potassium ferricyanide papers. This is a simple test that can be conducted on recently blast-cleaned steel. If there are any salts present on the surface, the paper will turn “prussian blue”. While this test does not quantify the amount of salt present, it does tell you if salts are present. A zero means good to proceed with the coating application, and a blue color means run further testing to quantify the amount of salts.
Note: The chemical in the paper reacts with the Fe++ so any salt anions present that react with the steel to form the Fe++ will react with chemical in the paper. This test only works on steel.
It should also be noted that not everything that is soluble can be tested for. Sugars and other organics, alcohols and solvents can all solubilize in water and will cause the same blistering as salts. Over my career, I believe have seen more blistering due to solvents than I have due to salts.
Using Surfactants to Remove Salts
To remove soluble salts, the best method is to clean the newly blasted surface with a large quality of high-pressure water. The higher the pressure, the more efficient the removal of contaminants. (Read the article Blast Pressure: How to Get It Right for more information.) There are salt removers on the market that can assist in removing salts. There are two main solutions in use and both work by reducing the surface tension of the water to better wet out the surface. One product consists of a 100% volatile, non-ionic tertiary amine. This product helps remove all salt ions from the surface and it evaporates off with the water. When the surface is dry, you are good to paint. The second product, consists of amines, ionic surfactants and acids to remove specific ions from the surface. Since it contains acid salts and ionic cleaners I recommend it be washed off the surface because it may cause contamination.
Summary
In any country except the United States, the issue of testing for soluble salts is settled: coating inspectors test for Total Salts in accordance with ISO test procedures. In the U.S., the coatings industry continues to argue and write confusing specifications.
Testing for Total Soluble Salts is not the same as testing for Specific Ions. Knowing the total salts on a surface tells us if that surface is contaminated and therefore more prone to develop osmotic blistering.