The short answer to this question is yes.
The presence of soluble salts, particularly sulfates and chlorides, at the paint/hull interface can have detrimental effects on the integrity of the coating system. These salts promote destructive behaviors such as osmotic blistering.
Osmotic blistering, as its name suggests, is blistering by way of a process known as osmosis. Osmosis is the movement of a solvent through a semipermeable membrane from a less concentrated solution to a more concentrated one. The process continues until the concentrations of solute on each side of the membrane are equal.
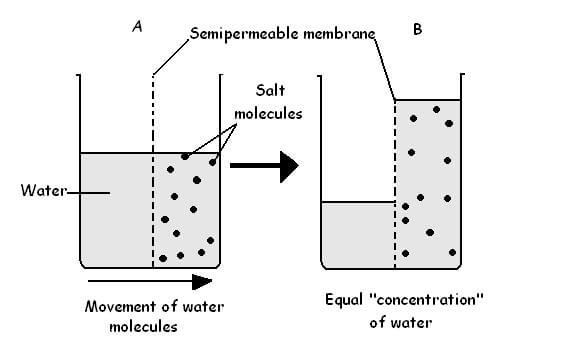
Figure 1. Process of osmosis. (Source: By Rlawson at English Wikibooks, CC BY-SA 3.0)
To simplify, what this means is that water (the solvent) will continue to move through the paint layer (semipermeable membrane) until the concentration of dissolved salts (solute) at the paint/hull interface is equal to the concentration of dissolved salts on the painted surface.
To better understand this definition and how it affects paint bonding, let’s look at the stages of osmotic blistering below.
The paint (typically Gelcoat) acts as a semipermeable membrane. In other words, it allows only some components to pass through. In this case, the coating permits moisture, but not salts, to penetrate. The foundation for osmotic blistering is set when the coating is applied to a surface that is contaminated with soluble salts. (Related reading: Field Methods for Extraction and Analysis of Soluble Salts.)
When the fiberglass or carbon fiber hull sits in the water, the osmosis process begins. The water permeates through the semipermeable coating to mix with and dissolve the soluble salts at the paint/hull interface.
Because the concentration of dissolved salts at the paint/hull interface is higher than that at the paint surface, diffusion of water through the coating continues. This process persists until the soluble salt concentration on both sides of the membrane (paint surface) are equal.
The water gathered at the paint/hull interface eventually exerts an upward force on the coating that is greater than the adhesion and cohesion forces, causing blisters to form.
While osmotic blistering is difficult to control on fiberglass and carbon fiber hulls, there are several treatment methods available. For example, applying a thick epoxy coating to the hull can create an extra separation layer between the Gelcoat and the water. This added protection helps to delay the onset of osmotic blistering.