What Does
Resistance Polarization Mean?
Resistance polarization is a part of electrode polarization arising from an electric current through an ohmic resistance within the electrode or the electrolyte. It results from the pure resistance elements along the current path in electrochemical cells.
The effect of resistance polarization may be significant where this current flows from the anode to the cathode in an electrolyte, depending on the resistance of the electrolyte.
Resistance polarization is observed either in the region of ionic conductivity or in the region of electronic conductivity.
Resistance polarization is also known as ohmic polarization.
Corrosionpedia Explains Resistance Polarization
Current is transported from the anode to the cathode by ions in the electrolyte and in the metallic path from the anode to cathode. Because of the normal high conductivity of metals, almost no resistance is offered to the current flow in the metallic path. However, resistance can be encountered if the distance between the anode and the cathode is appreciable.
The effect of resistance polarization on the corrosion current in an electrochemical corrosion cell are shown in below figure:
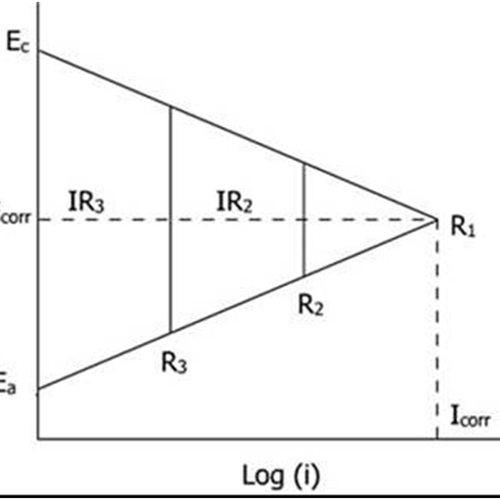
With seawater as the electrolyte, the anodic and cathodic polarization curve intersect at point R1, where the potentials of the anode and the cathode are polarized at the same value. If the resistivity of the solution is high, a potential drop (IR) results from the flow of the current in the resistive solution and the potential of the anode and cathode differ slightly in the case of seawater.
The reason is that the potential drop (IR) diminishes the driving force of activation polarization and the anodic and cathodic reactions are not polarized to the same potentials. This situation is represented by R2 in the above figure, and further increasing the electrolyte resistance, the magnitude of the ohmic drop would further increase, as shown by R3 in the figure. With an increasing resistance offered by the electrolytes, the magnitude of the corrosion current decreases as shown by R1, R2 and R3 in the figure.
Incidentally, increasing the solution resistance offers a good method of controlling corrosion, since decreasing corrosion current means decreasing corrosion rate.