Editor's Note:
The following is an abridged version of …. Smart Coatings and Nanotechnology Applications in Coatings article featured in ASM Handbook, Volume 5B: Protective Organic Coatings. This handbook is comprised of 480 pages and divided into five sections, which offer introductory material, an in-depth presentation of specific coating materials, practical information on surface preparation and coating application, coverage of coating use by various industries, and a detailed discussion of coating analysis and evaluation methods. Volume 5B authors provided the latest information on the many industry standards that must be adhered to in the preparation, application and testing of protective coatings.
For the full, in-depth article along with the rest of the authoritative, reliable sources of information, visit the webpage.
***
Ongoing research in the areas of nanotechnology and so-called smart coating or intelligent coating systems is showing great potential for the development of new high-performance coatings. (For more on this topic, read Coatings Advances: Nanoparticle Technology and Cold Sprays.)
Research Efforts on Nanotechnology and Nanocoatings
Nanomaterials research is undertaken in over 30 countries, with the United States, Japan, China, France, the United Kingdom, and Russia accounting for 70% of the world's scientific papers on nanotechnology. Today, numerous technical conferences are held worldwide on the topics of nanotechnology and smart coatings. Although most of this research remains in the laboratory, commercial applications are beginning to emerge. According to NANOfutures, the home of the European initiative for sustainable development using nanotechnologies, the global nanotechnology market hit $29 billion in 2010 and is forecast to grow to $498 billion by 2015, with coatings leading the increase. The value of the nanocoatings market is forecast to reach $20 billion by 2015. The number of households using nanotechnology is predicted to surpass 250 million by 2015. The National Science Foundation projects that nanotechnology will become a $1-trillion-a-year market globally by 2015.
In light of these growth forecasts, both coating engineers and end users should have a basic understanding of the terminologies, processes and materials involved. This article reviews current developments in these exciting new fields.
What is Nanotechnology?
Nanotechnology is the study of matter having physical dimensions on the scale of 1 to 100 nm. (A nanometer is one-billionth of a meter: 1 nm = 10-9 m.) To put things in perspective, the width of human hair is approximately 80,000 nm, and atoms have dimensions on the order of 0.1 to 0.3 nm.

Figure 1. Representative sizes and shapes of nanomaterials.
Nanotechnology applications within the coatings industry today include:
- The use of extremely small nanoparticles of matter as raw materials in coatings
- The development, in situ, of extremely fine nanostructures within coatings
- Coatings which themselves are extremely thin, such as deposited films (i.e., nanofilms)
Materials and structures having nanoscale dimensions are of great interest to science and industry because they have been found to exhibit very unique and unexpected material properties due to their relatively great surface area and resultant very high surface-area-to-weight/mass ratio compared to conventional engineering materials.
Understanding Smart Coatings as a Type of Nanocoating
Smart coatings, by definition, have a built-in intelligence mechanism that allows the coatings to respond to environmental stimuli. During service conditions, these coatings can change in some fashion so their appearance or protective capabilities are enhanced. (Read How to Plan Facility Coatings Condition Assessments to learn how to determine the environmental conditions the coating system will need to endure)
Environmental stimuli for smart coatings may be of a physical nature, such as impact, or of a chemical nature, such as pH changes. Typically, the coating becomes activated in some way by sensing environmental stimuli. A self-healing coating, for example, can be designed to release a crack-repairing liquid polymer when physically damaged, or a corrosion-resistant coating can be designed to release a corrosion inhibitor when sensing pH changes known to occur during active corrosion processes.
Nanotechnology and smart-coating technologies have been reported to show great promise for improved performance in a number of critical areas:
Coatings utilizing nanotechnology are not necessarily smart, and smart coatings may not necessarily utilize nanotechnology. However, there often is considerable overlap. Nanotechnology may provide a pathway for the development of advanced smart-coating systems by making new nanoscale raw materials available to coating formulators. Nanotechnology and smart coatings are complementary technologies in this respect.
Finally, the terms nanotechnology and smart are buzzwords today in the coatings industry and often are used too loosely. Caution is recommended when reviewing new products claimed to be based on nanotechnology or smart technology.
Comparing Smart Coatings with Conventional Coatings
There is considerable interest in the development of coatings systems that can respond to environmental stimuli in a smart or intelligent manner. Such coatings truly represent the ultimate in advanced coatings technology.
Many conventional coatings, such as architectural house paints, are relatively unsophisticated passive or barrier materials. These products are designed to protect substrates by acting as a physical barrier and/or to impart aesthetic qualities such as color, gloss and texture.
Functional coatings and equipment coatings, which represent the next step up in coatings technology, are coatings designed for more specialized applications, often through the use of specialized additives to improve key properties such as corrosion resistance, fire retardance, bacteria resistance and so on. For example, coatings often are formulated with slightly soluble corrosion inhibitors that can leach from the coating film over time to inhibit corrosion processes. In this example, release of corrosion inhibitors occurs regardless of whether or not corrosion is likely to occur.
 Figure 2. Functional coatings for corrosion prevention.
Figure 2. Functional coatings for corrosion prevention.
Smart coatings represent an advance over traditional functional coatings in that they are active materials that change in a tailored way to environmental stimuli, leading to enhanced performance. A functional coating containing slightly soluble corrosion inhibitors, for example, would be considered a smart coating if the release of corrosion inhibitors occurs only when corrosion activity is likely. The advantages of this would be obvious in terms of reduced inhibitor depletion and extended durability.
Environmental stimuli for smart coatings may be either of a physical nature, such as impact/damage, ultraviolet degradation, or surface wetting, or of a chemical nature, such as interactions with atmospheric gases, corrosion processes or solvents. Coating responses for smart coatings can include changes in color, inhibitor release, surface catalysis and so on.
Examples of smart coatings include:
- Self-healing coatings that contain polymer filled microcapsules. When the coating is damaged or cracked, polymer is released to physically repair the damage.
- Corrosion-inhibiting coatings that can chemically detect corrosion activity and release a corrosion inhibitor or change in some way to be more corrosion resistant
- Chemical-agent-resistant coatings that change color to signal the presence of chemical agents
- Tunable coatings for windows that control light transmission depending on the intensity of the light
The distinction between smart and functional coatings is an important one. Often coatings claimed to be smart would be better described as highly advanced functional coatings. As such, most research today is based on advanced functional coatings versus truly smart systems. However, the concept of developing coatings that can respond in a tailored way to environmental stress certainly is intriguing, and this will continue to be an active research area. (Related reading: Top Nano Coatings Developments to Know for Corrosion Protection.)
Corrosion of iron requires oxygen and water. Rusting is catalyzed by chloride ions. Thus, good barrier properties of a coating are mandatory. Cracking will totally destroy local anticorrosive properties. Oxygen and water will penetrate nanocracks without resistance and start corroding the surface. Self-healing coatings can regenerate surfaces after small cracks occur. An even better approach is to prevent the crack formation in the first place. Carbon nanotubes (CNTs) and other fibrous nanomaterials, such as silicon carbide whiskers, have been used for the purpose of enhancing the performance of coatings containing sacrificial metal. It is advantageous if the CNTs are functionalized and chemically bound with a coating polymer, such as epoxy resin.
Ultimately, despite CNTs and self-healing methods, coatings will be damaged to some degree. However, corrosion can be slowed dramatically by incorporating sacrificial metal particles into the composition. These metal particles should be in electrical contact with the substrate metal; that is, their concentration should exceed the percolation threshold, even when they have been partially consumed. In practice, this requires more than 60 vol% of sacrificial metal particles in a coating. The integrity of the coating will suffer, however, if it contains so high a concentration of solid particles. This problem has been solved partially by adding graphite for increased electrical conductivity, so that the amount of metal particles can be decreased.
Physical toughness and electrical conductivity can be optimized simultaneously by using CNTs in anticorrosion coatings. Metal particles provide cathodic protection for the substrate metal, while CNTs provide a conducting network so that all sacrificial metal particles protect the substrate. Simultaneously, the CNTs reinforce the coating so that cracking is diminished. Zinc is the optimal sacrificial metal for the protection of steel structures. Under certain conditions, nickel or cobalt may prevent rust formation, even when they do not prevent the initial oxidation of iron. The advantage of nickel and cobalt over zinc is they may last longer.
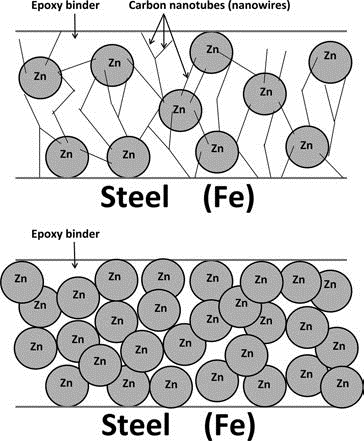
Figure 3. Diagram of carbon nanotubes connecting steel and zinc with an epoxy binder.
Carbon nanotubes have been used very successfully in epoxy coatings filled with sacrificial metals in corrosion-resistant coating applications. Although such coatings (e.g., zinc rich primers) have been used for many years and are considered to offer excellent performance, they can be brittle and porous due to the high loading of sacrificial metal needed to establish conductive pathways within the coating. The inclusion of CNTs in these coatings, as illustrated in Fig. 3, allows for the use of much lower levels of sacrificial metal, while conductivity and corrosion inhibition still are maintained.