When used properly, stainless steel enjoys a strong and enduring reputation for visual appeal and structural integrity in a wide range of applications and environments. But, like all materials, stainless steel may become stained or discolored over time, impairing the overall look. This brown discoloration – tea staining – has been identified in coastal applications.
This article provides information on tea staining and what fabricators, specifiers and end users should do to help avoid it and enjoy the long life and clean appearance of stainless steel. (Learn more about this metal in the article An Introduction to Stainless Steels.)

Figure 1. Stainless steel architectural components.
What is Tea Staining?
Tea staining is discoloration of the surface of stainless steel by corrosion. It is a cosmetic issue that does not affect the structural integrity or the lifetime of the material. Tea staining occurs most commonly within about five kilometers (3 miles) of the surf and becomes progressively worse closer to the marine source.
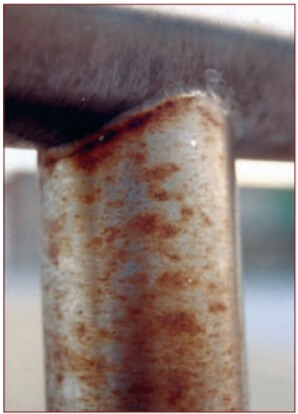
Figure 2. Tea staining of stainless steel.
However, wind exposure, pollution levels, local sheltering and higher temperatures can create environments where tea staining might occur 20 kilometers (12 miles) or more from the surf. The effect is much less severe around sheltered bays. These same factors also increase corrosion rates of alternative materials.
Other causes of staining that are not tea staining include carbon steel contamination, uncleaned welds and fumes from chemical such as hydrochloric acid or bleach.
Why Does Tea Staining Occur?
The relationships between the contributing factors are complex, but generally become increasingly critical closer to salty water. Tea staining occurs when local conditions (such as temperature, relative humidity and presence of corrosive substances on the surface) are too aggressive for that stainless steel grade in its installed condition.
There are important factors that promote the occurrence of tea staining that should be considered, as shown in Table 1 and explained below.

Table 1. Conditions Reducing the Risk of Tea Staining
1. Presence of corrosive substances
The presence of sea salt on the surface of the stainless steel is one of the major factors that cause tea staining. Sea salt has the characteristic of staying wet until a very low relative humidity (RH). The result of this is that the surface stays wet (and is corroding) longer with sea salt compared with sodium chloride. However, the presence of industrial pollutants could also make the conditions more aggressive.
2. Atmospheric conditions
A combination of atmospheric conditions with high humidity (e.g., tropical climates) and a high temperature creates worse conditions for the occurrence of tea staining. The high humidity generates a film of moisture that dissolves the salt deposits and creates a corrosive solution on the surface. The low humidity and absence of corrosive deposits means that tea staining is rarely a problem indoors. (Related reading: The 5 Factors of Atmospheric Corrosion.)
3. Surface orientation and design
Poor drainage promotes corrosion whether it is because the surface is nearly horizontal or has a texture that traps contaminants. Conditions are very aggressive in rain-sheltered areas such as the underside of sloping roofs, downpipes under eaves or in a building rain shadow. These can cause significant tea staining. Designs with corners or crevices (such as intermittent welds) can trap water and lead to more serious corrosion than tea staining.
4. Surface roughness
Deep grooves or metal folds on a surface are more susceptible to corrosion because they can trap salts (chlorides). When the surface dries the salts become concentrated, making the conditions more aggressive. A deeper groove will have more trapped water (and salts) so the bottom of the groove will be exposed to salt concentration above its resistance for longer — which will initiate corrosion. There is a critical surface roughness of approximately 0.5 μm Ra for cut or abraded surfaces. This is probably due to increased levels of grinding debris from coarse polishing. Abraded surfaces smoother than approximately 0.5µm Ra are much less susceptible to corrosion.
5. Surface characteristics
To achieve the best corrosion performance of a stainless steel, the surface should be clean, free of contamination such as carbon steel swarf or manganese sulfide inclusions, and have a continuous passive layer. Acid pickling, acid passivation or electropolishing for sufficient time will remove these contaminants from the surface as well as restore the passive layer, leaving the stainless steel with a clean and corrosion resistant surface. (These techniques are described in Using Pickling and Passivation Chemical Treatments to Prevent Corrosion.) If a stainless steel is welded, the heat input will locally destroy the passive layer and a dark non-protective oxide is formed around the weld. To achieve best corrosion performance and restore passivity of the weld, the heat tint and underlying chromium depleted layer must be removed. How this is done is described later.
6. Appropriate grade
There are several hundred grades of stainless steel with different chemical compositions but only about 10 in common use. All owe their corrosion resistance to the thin chromium oxide film on the surface, although other additions such as molybdenum and nitrogen can improve the corrosion resistance, especially in chloride containing environments.
A formula based on the content of these three elements is useful to rank the corrosion resistance of different grades. This pitting resistance equivalent (PRE) number is calculated by %Chromium + 3.3 %Molybdenum + 16 %Nitrogen. The PRE ranges from 10.5 for the grades with the lowest corrosion resistance to more than 40. For acceptable corrosion resistance, typically a PRE of approximately 18 is adequate away from marine influences; a PRE of approximately 24 is required for marine atmospheres while severe marine atmospheres may require a PRE of approximately 34. The higher the PRE, the greater the corrosion resistance.
7. Maintenance
Stainless steel is a low maintenance material but it is not generally maintenance free. A light and regular wash is best and natural rain washing may be sufficient. If not, then consider washing the stainless steel when you wash an adjacent window. Lower grades will require more regular maintenance and if the environment causes sticky deposits, a solvent and detergent mix may be required. Application of oils or waxes will temporarily restrict chloride access to the stainless steel but they need regular renewal. These temporary protectives also tend to attract debris and dull the surface.
Guidance in Fabrication
Design, fabrication and handling
Poor design and fabrication can lead to tea staining or more serious corrosion of stainless steels. Surfaces should be free draining, boldly exposed to rain washing and avoid channeling of runoff. Horizontal surfaces or curves that cause ponding are specific problems. Abraded surfaces should not be rougher than 0.5 µm Ra and the grain should be vertical to avoid ponding and collection of contaminants. For abraded surfaces, the best corrosion resistance will be achieved if a nitric acid passivation treatment is carried out as a final step.
Competent stainless steel fabricators will avoid carbon steel contamination (which can cause other corrosion problems), so choose designers and fabricators that are experienced with stainless steel to achieve the best outcome.
Appropriate grade selection
Each stainless steel has a limit to the concentration of salts that it can comfortably resist: the higher the alloying content (Cr, Mo and N), the higher the resistance to corrosion. Exposure of a particular grade of stainless steel to a more aggressive environment than it can resist will cause tea staining.
Grade 316, or a grade with equivalent corrosion resistance, should be selected as a minimum within five kilometers of the surf. For critical applications (e.g., splash zones, unwashed areas or rough surfaces), higher grades of stainless steel such as duplex or ‘super’ grades may be required.
The lower alloyed and less expensive grades (such as 304 or 430) will probably become tea stained or even suffer more severe corrosion in a marine environment.
Treatment of welds
Pickling after welding is one method of promoting good performance of stainless steel near the coast. This chemical treatment normally uses a mixture of hydrofluoric and nitric acids in a gel, paste or bath. It removes the welding oxide and chromium depleted layer underneath and rapidly restores the passive layer, which gives stainless steel its corrosion resistance. A darker heat tint means a thicker oxide and a longer exposure to pickling acids is required. Pickling removes material from the surface in a controlled way and may etch and dull the stainless steel surface. Excellent gas shielding, so there is no more than a pale straw color, may avoid the need for pickling provided the environment is mild. An alternative is to mechanically remove the scale and underlying chromium depleted layer, followed by a chemical passivation treatment using nitric acid. Any mechanical removal must not unduly roughen the surface – the limit being 0.5 μm Ra as mentioned previously.
Installation and inspection
After installation, the completed structure should be visually inspected for surface damage or contaminants. If contamination is suspected, several cycles of a misting and drying test with tap water are relatively simple. The sensitive ferroxyl test (described in ASTM A380) may also be used in critical applications. If discovered, imperfections should be removed and the corrosion resistance chemically restored by pickling or passivating treatments or by electropolishing.
Do not use hydrochloric acid
Hydrochloric acid, sometimes used to clean cement or mortar residues, must not be used on stainless steel — it will stain the surface and usually start more serious corrosion.
Key Design Recommendations
Plan to get the desired result
Marine environments are the most aggressive for all building materials.
Stainless steel’s corrosion resistance in marine environments means that installations are likely to remain structurally sound for decades.
It must be recognized, however, that keeping a pristine surface finish requires understanding and, usually, additional cost. Determine your expectation of the structure and plan ahead to achieve and maintain the intended result. This normally includes a maintenance program.
Environment
Tea staining is most likely to occur up to five kilometers from a surf beach and one kilometer from still marine waters. There is no hard and fast rule: wind and weather conditions play a big part and the severity of the conditions increases sharply as you approach the surf. AS 2312 suggests that in some special circumstances, 20 kilometers from the coast can still constitute a marine environment. AS 4312 has corrosivity maps that may assist in decisions. The closer to the source of salt, the more critical it is to follow the recommendations in this article.
Areas that are sheltered or not rain-washed are particularly susceptible. Tropical and high humidity areas are also more at risk of tea staining.
Specify and insist on a smooth and clean surface finish
To minimize the risk for tea staining the smoother the surface the better. A surface roughness of less than 0.5 µm Ra is strongly recommended for abraded finishes. Surfaces smoother than 0.5 µm Ra will have even better corrosion resistance. The most corrosion resistant, mechanically finished surface is a mirror polish (ASTM A480 No.8 or EN10088.2 class 2P). It is very smooth, resistant to salt accumulation and easy to clean. The surface roughness of a mirror-polished surface is so low that it is not reliably measurable by mechanical (stylus) instruments.
A No.4 finish to ASTM A480 just means an abraded (linished) finish. Specifying a No.4 finish is inadequate without indicating the required roughness.
The Euronorm standard EN10088.2 (finish 2K) recognizes this and requires Ra<0.5µm but also that the abraded profile is clean cut.
Components used near the sea can be made more resistant to tea staining if they are passivated to remove surface contaminants such as steel smears, weld spatter or sulfide inclusions. Mild levels of contamination may be removed by nitric acid passivation, which should not change the surface appearance although it may slightly cloud a mirror polish. More severe contamination by particles of steel or grinding debris may require pickling, which etches and usually dulls the surface. Either process may use pastes or gels (which can be applied on site) or liquids in baths in a factory. These chemical processes take longer if it is cold.
Electropolishing has been found to be extremely effective in removing surface contamination and passivating the surface. It also brightens and slightly smoothes the surface as well as rounding sharp edges and removing the peaks left from polishing operations. Electropolished surfaces have a characteristic luster but may not be mirror smooth. A mechanically mirror polished surface will normally lose its mirror reflectance if electropolished.
Smoother mill finishes such as 2B and bright annealed (BA) are widely available in flat products. Provided they are not damaged during fabrication, they offer good resistance to collection of salt deposits and hence to tea staining.
Rolled embossed finishes may be suitable for some applications. These have very smooth surfaces but with a pattern that lowers reflectivity. Think carefully about the pattern and how it will be oriented — avoid pools of water sitting on the surface.
Specify and insist on the right grade
In marine environments, use grade 316 or one with equivalent corrosion resistance unless a higher grade is required because the job is aesthetically critical and regular maintenance is unlikely.
Where there are high aesthetic expectations a number of more corrosion resistant stainless steel grades can be considered. The first step up from 316 is 2205 and then the super duplex grades, although the high molybdenum austeniticsand high molybdenum ferritics may also be useful. Smooth surface finish and maintenance are still important with these grades.
Treatment of welds
For general architectural applications welds should comply with applicable standards. However, this specification does not guarantee the absence of structurally minor surface defects that can act as traps and corrosion initiating sites. The protruding weld can be ground flush, and good resistance to tea staining achieved (a Grade I finish) when polished to 320 grit or finer finish. The smoother the surface, the better the tea staining resistance. Passivation will occur in chloride-free, moist air within a day. Chemical passivation treatment with nitric acid may be applied to:
- Substantially reduce the time required for passivation
- Provide a more corrosion resistant passive film
- Remove possible iron contamination
- Dissolve exposed manganese sulfide
Chemical passivation must be applied after abrasion if the environment is particularly aggressive.
An alternative cleaning treatment is a Grade II blast cleaned finish. This will require a post blasting passivation treatment. The blasting should remove heat tint and the chromium-depleted layer but not make the surface roughness worse than 0.5 µm Ra, must not leave folds or crevices and should not embed corrodents. The Grade II stainless steel wire brushing treatment is not adequate to control tea staining.
Where a polished (or linished or ground) finish is desired, abrasives should be used with lubrication if possible. In selecting abrasives, consideration should be given to matching the surrounding finish.
A Grade II pickled finish will provide good tea staining resistance without grinding the weld flush, provided there are no significant surface crevices or defects. Where linishing or blasting is not performed, pickling of site welds (using mixed hydrofluoric plus nitric acids) should take place as a final step in the weld procedure.
Pickling will remove any fabrication contaminants and restore the passive chromium oxide layer, resulting in a corrosion-resistant surface. Electrocleaning has been used instead of pickling to remove weld scale and heat tint, especially when hydrofluoric acid use is restricted. While passivation treatments do not normally affect appearance, pickling treatments are likely to dull bright surface finishes. Electropolishing is also a very effective method of passivation. The ASSDA Reference Manual describes these treatments in more detail.
Specify and insist on regular maintenance
Washing removes deposits (such as salt) that can cause corrosion. It is necessary to avoid tea staining. Rain-washing the surface is helpful in reducing tea staining, so design the job to take advantage of the rain, but ensure good and even drainage.
Stipulate that the stainless steel also be washed when cleaning of the surrounding area takes place. As a guide, stainless steel should be washed if a window requires washing. For best results, wash with soap or mild detergent and warm water followed by rinsing with clean cold water. The appearance of the surface can be improved further if the washed surface is wiped dry.
If routine cleaning of the surrounding area does not take place, washing frequency for the stainless steel is recommended as in Table 2 below.
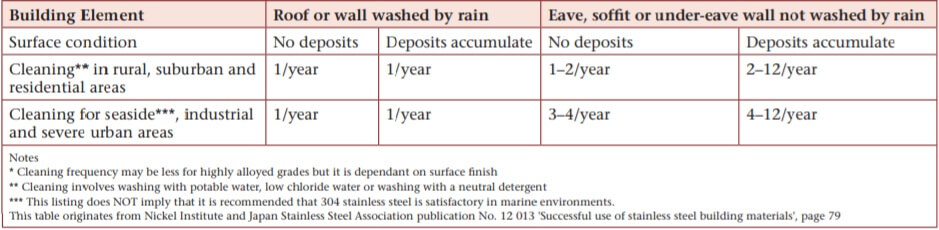
Table 2. Stainless steel washing frequency recommendations.
It is essential that abrasive cleaners or those containing chlorides or bleach are NOT used to clean stainless steels as they will damage the surface. If some tea staining does occur, then an assessment of the 7 points is required to determine why the problem occurred. Simple mechanical polishing is unlikely to both remove current and prevent future tea staining. Reasonably simple chemical cleaning and passivation is usually the most effective treatment.