What Does
Glass Transition Temperature (Tg) Mean?
A glass transition temperature (Tg) is the temperature at which a polymer turns from a ductile material to a hard, brittle material. It is the temperature at which carbon chains start to move. At this stage, the amorphous region experiences a transition from a rigid state to a flexible state with the temperature at the border of the solid state changing it to more of a viscoelastic (rubbery) one. At this temperature the free volume, or the gap between the molecular chains, increases by 2.5 times.
The viscoelastic properties of a semi-crystalline polymer allow flexibility as is the case of packaging materials.
The glass transition temperature is a property of the amorphous portion of a semicrystalline material. At the point where the ambient temperature is below Tg, the molecules of amorphous materials remain frozen in place and behave like solid glass. Plastic materials have a lower Tg, although plastic materials whose molecular structure is stiff and rigid show a higher Tg.
Every polymer with an amorphous structure has its own unique glass transition temperature, which is a useful factor in determining whether a given material is better suited for flexible or rigid applications.
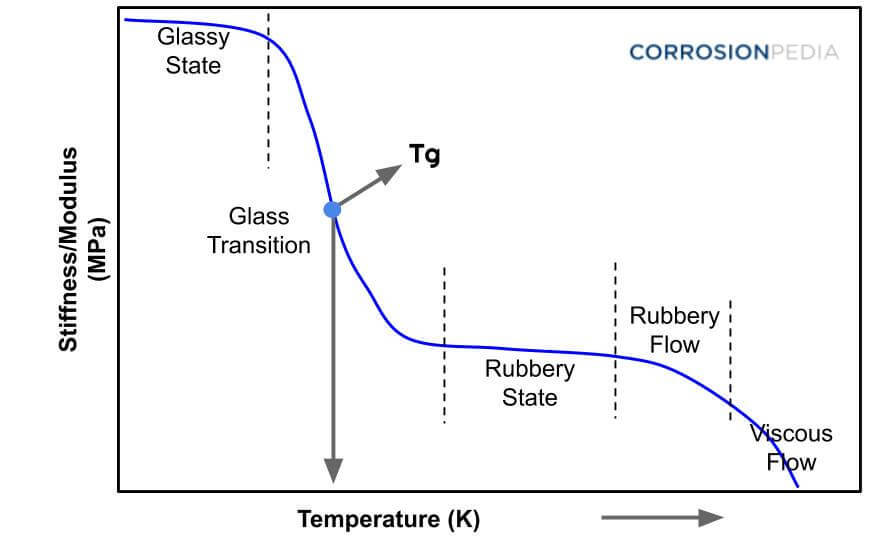
Figure 1. Graph of glass transition temperature plotting the temperature and stiffness of a material.
Corrosionpedia Explains Glass Transition Temperature (Tg)
The temperature at which an amorphous polymer material turns into a viscous liquid or rubbery form when heated is known as the glass transition temperature (Tg). It can also be defined as a temperature at which an amorphous polymer develops the characteristic glassy-state properties such as brittleness, stiffness and rigidity upon cooling. This temperature can be used to identify polymers.
Also, at the Tg, the main backbone chain mobility changes. At lower temperatures there is still molecular motion but the main backbone chain is frozen in place. The Tg for a given plastic can be changed by the incorporation of a plasticizer, as is the case for PVC.
The value of Tg depends heavily on the mobility of the polymer chain, and for most synthetic polymers lies between 170°K and 500°K (-103°C and 227°C).
Pure crystalline polymers do not have a glass transition temperature because the glass transition temperature is only applicable to amorphous polymers. Pure amorphous polymers do not have a melting temperature; they only have a glass transition temperature. However, many polymers are composed of both amorphous and crystalline structures. This means that many polymers have both a glass transition temperature and a melting temperature. The glass transition temperature is lower than the melting temperature.
Practical Applications of the Glass Transition Temperature (Tg)
The different glass transition temperatures of different polymers make various polymers better suited for some applications than others are. For instance, a rubber tire for an automobile is soft and ductile because at normal operating temperatures it is well above its glass transition temperature. If its glass transition temperature were greater than its operating temperature, it would not have the flexibility required to grip the pavement.
Other polymers are designed to operate below their glass transition temperature. An example of this is a stiff plastic handle on a tool. If the plastic handle were to have a glass transition temperature below its operating temperature, it would be too flexible to allow one to grab it and effectively use the tool.
Factors Affecting the Glass Transition Temperature
External factors, like humidity or moisture level, may also affect Tg. Because moisture tends to diffuse slowly through a material, it may act as a plasticizer and cause the material to reach an equilibrium moisture content based on the relative humidly of the exposure. This results in a lower Tg. Materials used in an office environment will only pick up moderate amounts of moisture during their service life, compared to materials kept outdoors in a humid environment. Because of this, a lower drying temperature (well below the curing temperature) or controlling the moisture exposure may be appropriate.
How Glass Transition Temperature Testing is Performed
The classical way of measuring the glass transition temperature is to perform a series of mechanical tests over the expected temperature range. While there are several options for the test type, flexural strength or shear strength testing are the standards. Results are reported as a plot of flexural modulus or shear modulus in relation to temperature. The Tg is indicated where there is a significant drop off in material strength.
The most standard thermal methods for determining transition temperature are differential scanning calorimetry (DSC), dynamic mechanical analysis (DMA) and thermomechanical analysis (TMA).
A Practical Application for the Glass Transition Temperature
Epoxy coatings are used extensively for pipeline protection in the oil and gas industries. An important consideration is choosing the best epoxy formulation that provides efficiency and sustainable corrosion protection, especially in higher temperature conditions. The coating’s performance depends on plasticized Tg values.