What Does
Applied Current Mean?
Applied current refers to the logarithm of current density. It is used in determination of corrosion current density and corrosion potential as well as corrosion rate measurement.
When reaction mechanisms for the corrosion reaction are known, the corrosion currents can be calculated using Tafel Slope Analysis. The Tafel equations predict a straight line for the variation of the logarithm of current density with potential.
In anodic protection, the applied current is usually equal to the corrosion rate of the protected system. Thus, anodic protection not only protects, but also offers a direct means for monitoring the corrosion rate of a system.
Corrosionpedia Explains Applied Current
The corrosion current density and corrosion potential are determined from the Tafel plots of potential versus logarithm of corrosion current density, i.e. applied current. The linear polarization resistance (Rp) is typically calculated from the slope of a polarization curve where:
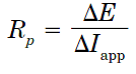
And where  E is the voltage change for an applied current (
E is the voltage change for an applied current ( Iapp). Rp itself can be converted in a corrosion current (Icorr) using the Stern-Geary approximation.
Iapp). Rp itself can be converted in a corrosion current (Icorr) using the Stern-Geary approximation.
The current density required for complete protection depends on the metal and on the environment. The applied current density must always exceed the current density equivalent to the measured corrosion rate in the same environment. Hence, the greater the corrosion rate, the higher must be the impressed current density for protection.
When the corrosion rate is cathodically controlled and the corrosion potential approaches the open-circuit anode potential, the required current density is only slightly greater than the equivalent corrosion current. But for mixed control, the required current can be considerably greater than the corrosion current, and it is still greater for corrosion reactions that are anodically controlled.
In cathodic protection, the applied current density has to be controlled in concrete. If the current magnitude is excessive, several problems can arise, such as reduction in bond strength, softening of the cement paste around the rebar steel, and cracking of the concrete.