There are many types of corrosion damage, such uniform corrosion and pitting corrosion that people can readily see with the naked eye. However, some corrosion damage is not visible while still being detrimental to the structure's or equipment's integrity. This article will take a closer look at one of the less visible corrosion damage types called intergranular corrosion (IGC), with a focus on developing a deeper understanding of how intergranular corrosion occurs, what materials at affected, the types of industries where intergranular corrosion typically occurs, and how to detect and mitigate the damage.
What is Intergranular Corrosion (IGC)?
Intergranular corrosion (IGC), sometimes referred to as intergranular attack (IGA), is a preferential or localized corrosion proceeds alone the grain (crystal) boundaries or immediately adjacent to the grain boundaries. In contrast, the majority of the grains remain mostly unaffected.
Although metal loss is minimal, IGC can cause the catastrophic failure of equipment. IGC is a common form of attack on alloys in the presence of corrosive media that results in the loss of strength and ductility. One should not mistake IGC with stress corrosion cracking (SCC). SCC requires stresses (residual or applied) to act continuously or cyclically in a corrosive environment producing cracks following an intergranular path.
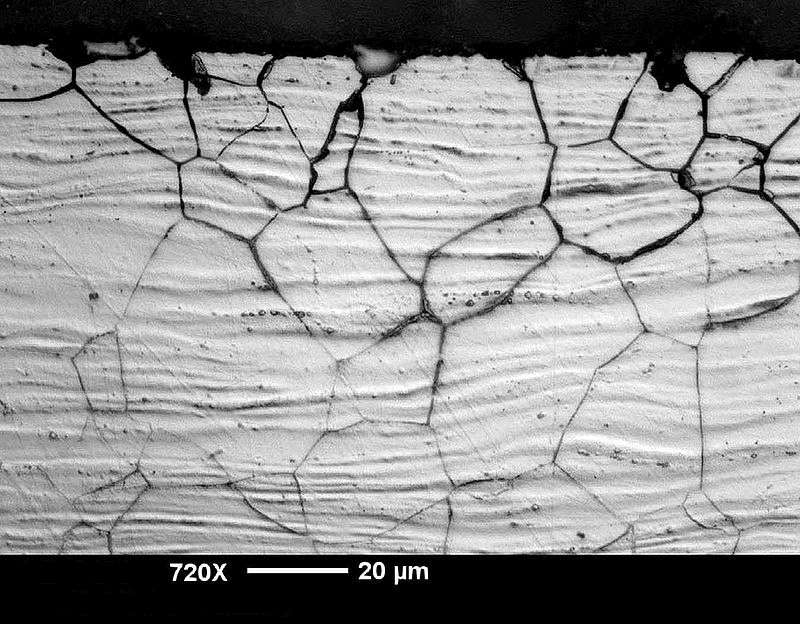
Figure 1. Intergranular corrosion attack in austenitic cold rolled stainless steel sheet. (Source: Antkyr, Creative Commons ShareAlike 3.0 Unported (CC BY-SA 3.0).)
The ICG localized corrosion at grain boundaries is caused by the anodic dissolution of areas weakened by the alloying elements, second phase precipitation or regions with isolated alloying or impurity elements. The remaining part of the exposed surface typically functions as the cathode, and large cathodic areas support the anodic dissolution process.
The cathode to anode ratio is generally greater than one. It depends on factors such as the volume fraction and distribution of electrochemically active phases, the distribution of detrimental alloying and impurity elements, and grain size.
The corrosion rate is dependent on the dominant corrosion mechanism, and factors such as the diffusion of species to or from the anodic front can govern the dissolution kinetics. A significant characteristic of IGC is the development of a relatively homogeneous and uniform depth of attack. The dissolution of grain boundaries causes the dislodging of grains, often referred to as grain dropping. Grain dropping is responsible for most of the weight loss observed after IGC exposure, and corrosion rates can therefore be several orders of magnitude higher than during general corrosion.

Figure 2. A stainless steel that corroded near a weld's heat affected zone (HAZ). (Source: NASA Corrosion Engineering Laboratory.)
Materials Commonly Affected by Intergranular Corrosion
Intergranular corrosion attack is mainly prevalent in certain types of stainless steel rather than in carbon steel. (Related reading: Why Stainless Steel is Corrosion Resistant.) However, the following materials are not excluded from IGC attack.
- Unstabilized austenitic stainless steel grades 304 and 316 used in chemical plants are prone to IGC attack when used in the sensitized stage. The sensitization is caused by the precipitation of chromium carbide at the grain boundaries in a zone adjacent to welds, where the temperature has been between 500 – 800°C (932 – 1472°F). (For more on this subject, read How Hot Shortness and Welding Affect Corrosion in Metals.)
- Nickel-copper alloys (Alloy 400, UNS N04400) are prone to IGC attack when exposed to certain types of hydrofluoric acid and chromic acid solutions.
- IGC attack can occur in nickel-molybdenum alloys (Alloy B, UNS N10001) exposed to hot hydrochloric and sulfuric acid due to the precipitation of molybdenum-rich constituents.
- Nickel-chromium alloys such as Alloy 600 are prone to IGC attack. Therefore, it is not intended for use in service in corrosive environments.
- Aluminum grades 2024 and 7075 are prone to IGC attack because CuAl2 precipitates at the grain boundaries that act as cathodes, accelerating the depleted zone adjacent to the grain boundary. Additionally, aluminum grades 5083 and 7030 are also susceptible to IGC attack.
- Zinc (Zn) of high purity is not prone to IGC. However, aluminum as an alloying element or impurities in the zinc alloy could cause an IGC attack.
Intergranular Attack of Austenitic Stainless Steels
With austenitic stainless steels, intergranular attack is usually the result of chromium carbide precipitation (Cr23C6) at grain boundaries, which produces a narrow zone of chromium depletion at the grain boundary. This condition is termed sensitization (Figure 3). Sensitization involves the precipitation of chromium carbides at grain boundaries, which results in a narrow zone of chromium depletion at the grain boundary.
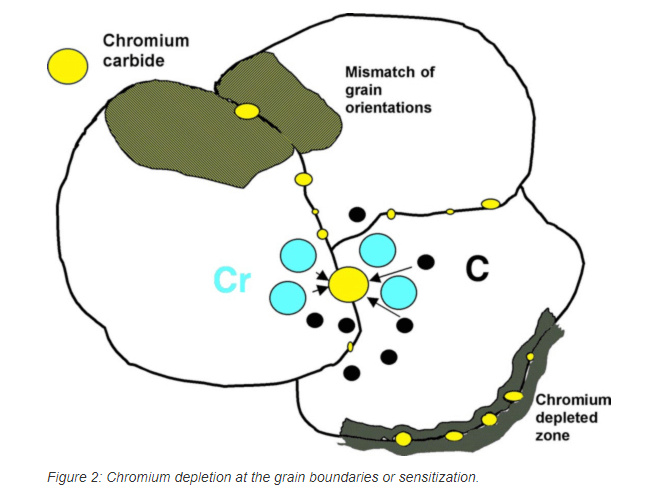
Figure 3.
Because the chromium is the primary alloying element that makes stainless steel corrosion resistant, the chromium-depleted regions are susceptible to preferential corrosion attack. It is believed that this occurs because the chromium content immediately adjacent to the carbide may be below that required for the stainless steel alloy. If the carbides form a continuous network on the grain boundary, then corrosion can produce a separation or gap at the boundary and possible grain dropping or loss.
Methods to Detect Intergranular Corrosion
Usually the IGC proceeds along grain boundaries and is hard to detect with the naked eye or any other non-destructive inspection technique. However, the material can be tested for resistance to IGC before fabrication of the equipment with specific laboratory methods, such as the Huey test (which uses a nitric solution) or the Strauss test to identify the susceptibility of stainless steel to intergranular corrosion. The Streicher test can also be used, which is based on a quantitative weight loss determination. Additionally, IGC cracking can be seen when a sample from the failed area is metallographically prepared and examined under a scanning electron microscope (SEM).
Mitigation Methods to Prevent IGC Attack of Austenitic Nickel-Chromium Stainless Steel
Conducting proper annealing and quenching treatments at the fabrication shop or mill will reduce the susceptibility of stainless steel and nickel-rich chromium-bearing alloys to IGC. When these treatments are successfully performed, dissolved chromium carbides, nitrides and molybdenum carbides, and their pre-precipitation forms, keep them in solution during the quenching.
In ferritic stainless steels (AISI Type 430, Type 446), the diffusion rate of carbon is so great that the precipitation of chromium carbides cannot be prevented, even with rapid water quenches from high-temperature annealing treatments. However, the rate of chromium diffusion in these alloys is also high. It is possible to restore the chromium-depleted zones surrounding the chromium carbide precipitates with heat treatments near 816°C (1,500°F). The result is a microstructure that contains extensive amounts of carbide residue, which is immune to IGC.

Figure 4. The heat treatment of pipeline welds to prevent intergranular corrosion. (Source: Berkut34 | Dreamstime.com)
When stainless alloys are welded, the formation of chromium carbides and nitrides can be prevented in many cases by reducing the carbon and nitrogen content. The introduction of the argon-oxygen decarburization process, vacuum melting, and consumable arc remelting have greatly influenced preventing chromium carbides and nitride formation in the alloys AISI Type 304L, Type 316L, Alloys C-276 and C-4, and the Fe-29%Cr-4%Mo.
The formation of chromium carbides in stainless steel can be prevented by adding the elements titanium (Ti) or Niobium (Nb). (Related reading: The Role of Chromium in Intergranular Corrosion.) These elements combine with carbon and lower its concentration such that chromium carbides are not formed during exposure in the sensitizing range of temperatures during welding and stress relieving, and even under operating conditions. These are called stabilized alloys, and they are AISI Type 321 (Ti), AISI Type 347 (Nb), Alloy 20Cb-3(Nb), Alloy 625 (Nb) and Alloy 825 (Ti).
Different sizes of weldments and other welding techniques (such as lower heat inputs) can reduce the degree of sensitization. However, it isn't easy to maintain definite control to make this approach generally applicable.
Industries that are Often Affected by Intergranular Corrosion
IGC can occur on any equipment fabricated from austenitic stainless steel, nickel-copper alloy, nickel-molybdenum alloy, nickel-chromium alloy, aluminum alloy and zinc alloy in any industry where the right conditions exist, which means that if the material has not undergone proper heat treatment and contains a higher carbon content (C > 0.03%) then it is susceptible.
Intergranular corrosion can lead to catastrophic failure in most process equipment if correct material and proper heat treatment haven't been used during the fabrication stage. The loss of cross section thickness and the introduction of cracks can have severe consequences for applications like pressure containment.