The refining of crude oil into usable products is a complex process with many opportunities for corrosion to gain a foothold. Understanding the typical atmospheric distillation process flow in a crude oil refinery is a valuable tool to help identify corrosion and implement controls in those locations where corrosion is likely to occur.
Upstream, Midstream and Downstream Sectors of the Oil and Gas Industry
The oil and gas industry is classified into downstream, midstream and downstream sectors. The upstream sector involves the exploration and production of oil and gas, the midstream involves the transportation of the produced oil and gas, and the downstream sector covers the processing and marketing.
Understanding Oil: Sweet and Sour, Heavy and Light
Crude oil is called "sour" when it contains unacceptable amounts of sulfur compounds, while "sweet crude oil" is the opposite. Crude oil is termed "heavy" when it is made up of longer hydrocarbon chain compounds, and "light" when it contains shorter chained hydrocarbons (light diesel to methane). Typically, crude oils are made up of paraffins (alkanes), naphthenes (cycloalkanes), aromatic hydrocarbons and asphalts.
An Overview of Crude Oil Refining
Crude oil refining is the key chemical engineering process of the downstream sector because it involves the production of gaseous and refined liquid products for the end user. The most common products are methane, liquefied petroleum gas (LPG), gasoline, naphtha, aviation kerosene, diesel, fuel oil and heavy residue. Figure 1 shows a typical atmospheric distillation process flow in a crude oil refinery.
Figure 1. Process flow diagram of a typical atmospheric distillation process in a crude oil refinery.
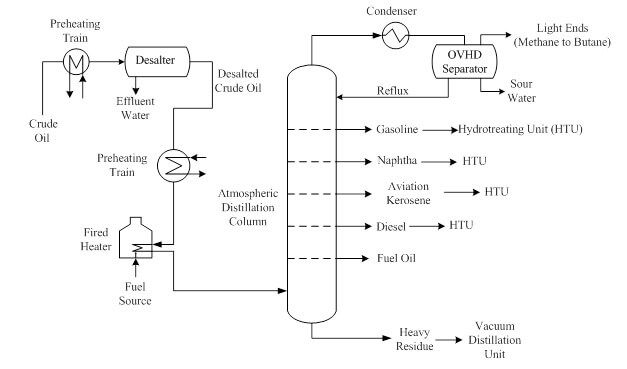
The crude oil from the tank farm (top-left in Figure 1) is first slightly heated before sending it to a desalter to remove most of the salt content. Downstream of the desalter is the crude oil preheating train where the crude oil is preheated prior to entering the fired heater. The crude oil is usually preheated with the distillates from the distillation column because this promotes heat integration. Fuel oil or surplus fuel gas from the refinery is usually used to provide the heat necessary for the fired heater. The fired heater raises the crude oil temperature to the atmospheric distillation temperature of 350°C to 400°C (662°F to 752°F). The two phase crude oil is sent to the bottom of the distillation column where different fractions distill along the column height based on the differential boiling point. The trays in the distillation column allow the distillates to be collected and drawn off, and then sent to either a storage tank or further processing (e.g., hydrotreating). The heavy residue, which are hydrocarbons with more than 25 carbon atoms drops to the bottom of the column and is sent to the vacuum distillation unit. The lightest hydrocarbons with only one to four carbon atoms leave the top of the distillation column along with water vapor and impurities. The vapor stream is condensed to recover the water vapor, which is then refluxed back to the column to enhance the distillation and separation process.
Crude Oil Corrosion Sources
Crude oil on its own is not corrosive, but the reservoir source and method of production may contain any of several impurities, such as water, salts, sulfur compounds, oxygen compounds and carbon dioxide (CO2). (Learn more in The 6 Corrosive Components That Can Be Found in Crude Oil.)
Salts and Water Content
The most common salts seen in crude oils are sodium chloride (NaCl), calcium chloride (CaCl2) and magnesium chloride (MgCl2). However, the salt concentration of crude oils depends heavily on the type and source of crude oil. Additionally, crude oil contains some free water which is usually removed by a surface separator.
The presence of these salts and water in the crude oil leads to the formation of hydrochloric acid known as salts hydrolysis according to Eqs. (1) to (4).
Eq. 1: CaCl2 + H2O ↔ CaO + 2HCl
Eq. 2: CaCl2 + 2H2O ↔ Ca(OH)2 + 2HCl
Eq. 3: MgCl2 + H2O ↔ MgO + 2HCl
Eq. 4: MgCl2 + 2H2O ↔ Mg(OH)2 + 2HCl
The salts hydrolysis usually occurs at 120°C and 210°C (248°F and 410°F) for MgCl2 and CaCl2 respectively and is more prevalent in regions of high temperature (from the preheating exchanger to the distillation column).
Additionally, during the cooling down of the distillation column light ends, the hydrogen chloride gas mixes with the steam condensate to form hydrochloric acid. This acid accelerates both general corrosion and pitting corrosion. Corrosion related to hydrochloric acid is difficult to control because the chlorine atom (with a very small size) easily penetrates the protective layers of surface metals.
Therefore, the acidity of the condensed water vapor (water condensate) in the overhead separator should be monitored regularly to determine if neutralization is required as well as to change the rate of alkaline injection. When this is established, organic neutralizers are injected at the vapor line (top exit) of the atmospheric distillation column to reduce the concentration of protons (H+). The most frequently used neutralizers, which are alkaline in nature, are ammonia (NH3) and filming amines (RNH2), and they react with hydrogen chloride to form ammonium chloride (NH4Cl) and amine hydrochloride (RNH3Cl) salts according to Eqs. (5) and (6).
Eq. 5: NH3(g) + HCl(g) → NH4Cl(s)
Eq. 6: RNH2(g) + HCl(g) → RNH3Cl(s)
The rate of neutralizer injection should be performed with precision to avoid a pH swing. This is because the pH stability is important towards maintaining a corrosion free environment. The amine hydrochloride salts should not be allowed to accumulate for a long time at the bottom of the overhead separator because it can trigger corrosion.
Sulfur Compounds
Crude oil and distilled fractions contain certain amounts of sulfur compounds and they are mostly bonded with carbon atoms, while the remaining are in the form of hydrogen sulfide (H2S) and elemental sulfur. During atmospheric distillation, H2S exits with the light ends and water vapor from the top of the distillation column. This not only attacks the internals of the distillation column but corrodes the downstream piping that conveys the light ends to the condenser and overhead separator. The corrosion also extends to light ends downstream processing equipment. Additionally, some of the H2S and other sulfur compounds also exit with liquid fractions such as gasoline, naphtha, aviation kerosene and diesel, causing severe corrosion problems.
In order to minimize corrosion issues at the top section of the distillation column and in downstream equipment, NH3 and/or filming amine is injected at the top exit (vapor line) of the distillation column. The reaction between NH3 and H2S leads to the formation of ammonium bisulfide, while the H2S and filming amine reaction forms amine bisulfate (See eqs. (7) and (8)). The bisulfate and bisulfide is withdrawn with the wastewater sent to the sour water treatment section.
Eq. 7: NH3(g) + H2S(g) ↔ (NH4)SH(s)
Eq. 8: RNH2(g) + H2S(g) ↔ RNH3HS
It is important to note that the ammonium bisulfide and amine bisulfate also introduce corrosion problems in the sour water treatment units. However, these sulfides and sulfates are less corrosive than H2S and their corrosion rates are greatly influenced by the fluid velocity.
As for the liquid fractions (gasoline, naphtha, aviation kerosene, diesel) containing H2S and other sulfur compounds, they are usually purified through a hydrodesulfurization process. The other sulfur compounds include but are not limited to mercaptan (RSH), sulfide (RSR), disulfide (RSSR), and thiophene (C4H4S), where ‘R’ in the chemical formula is a hydrocarbon compound. In the hydrotreating unit, pure H2 is used to desulfurize the liquid fractions as shown in Eqs. (9) to (12).
Eq. 9: 2RSH + H2 ↔ 2RH + 2H2S
Eq. 10: RSR + H2 ↔ 2R + H2S
Eq. 11: RSSR + H2 ↔ 2R + 2H2S
Eq. 12: C4H4S + H2 ↔ C4H4 + H2S
Single compounds containing both aromatics and sulfur (AS) are also present in crude oil distillates. The aromatic compound is ‘A’ while the sulfur compound is ‘S’. In such scenarios, hydrodesulfurization is also used to remove the sulfur compounds as shown in Eq. (13).
Eq. 13: AS + H2 ↔ A + H2S
Oxygen Compounds
Oxygen gearing hydrocarbons also exist both in crude oil and in the fractionated crude oil distillates. Naphthenic acids (RCOOH) are common oxygen-bearing hydrocarbons, where R are mainly cyclopentane and cyclohexane derivatives. Most naphthenic acids have an atomic mass ranging from 120 to more than 700. Naphthenic acids are usually present in crude oil distillates that boil from 200°C to around 370°C (392°F to 698°F), and at temperatures above 370°C (392°F) and 400°C (752°F) the acids will thermally decompose.
The presence of naphthenic acids in crude oil is determined by the total acid number (TAN) technique. In this technique, the amount of potassium hydroxide (KOH) in milligrams that neutralizes one gram of crude oil quantifies the TAN. Crude oils are also said to be corrosive when their TAN is greater than 0.5. Based on TAN results, corrosion prevention techniques will be put in place.
Corrosion due to naphthenic acids follows the reaction shown in Eq. (14), producing naphthenates and hydrogen. This reaction gradually eats away the surface area of the metal, hence increasing in corrosion rate.
Eq. 14: Fe + 2RCOOH ↔ Fe(RCOO)2 + H2
During the hydrotreating process to remove sulfur compounds from crude oil distillates (Eqs. (9) to (12)), hydrogenation of naphthenic acids also occurs (Eq. (15)). This hydrogenation reaction produces the corresponding hydrocarbon and water (steam).
Eq. 15: RCOOH + 3H2 ↔ RCH3 + 2H2O
Carbon Dioxide (CO2)
Another component that makes crude oil and distillates corrosive is the presence of CO2. The amount of CO2 can vary from reservoir location to location, most especially when CO2 enhanced oil recovery is applied. The dissolved CO2 in the crude oil leads to excessive corrosion especially in the presence of free water (Eq. (16)). This reduces the metal thickness (most cases carbon steel) after a period of interaction with CO2. Apart from the concentration of CO2 dissolved in the crude oil, the pressure of the crude oil also increases the corrosion rate. This is because the higher the crude oil pressure, the higher the CO2 partial pressure.
Eq. 16: Fe(s) + CO2(aq) + H2O(l) ↔ FeCO3(s) + H2(g)
The formation of iron(II) carbonate (FeCO3) can precipitate out of the solution and form a protective layer on the metal surface, hindering further corrosion. However, a continuous buildup of the FeCO3 can cause a pressure drop along the crude oil pipeline, which leads to further challenges. Additionally, the precipitated FeCO3 can be disturbed and washed away by the high pressure crude oil, leading to further corrosion of the metal surface. (Further reading: An Intro to Pipeline Corrosion and Coatings.)
In order to combat such problems in crude oil pipelines, appropriate material selection (stainless steel) should be used. On the other hand, most of the CO2 with the crude oil exits the top of the atmospheric distillation column with the light ends. Therefore, CO2 capture using reactive amine solvents are used to remove CO2 from the gas stream. In such a case, the amine solvent removes both CO2 and H2S from the gas stream (acid gas removal unit) before fractionating them into methane (demethanizer), ethane (deethanizer), propane (depropanizer) and butane (debutanizer).
Conclusion
The presence of sulfur compounds, oxygen compounds, salts and water leads to excessive corrosion problems in crude oil pipelines and downstream distillates equipment. Therefore, it is imperative to reduce the concentration of these impurities.
The interaction between salts and water is a key contributor to corrosion in crude oil pipelines because of hydrochloric acid formation. The use of ammonia and filming amines has the ability to reduce the associated problems mostly at the top exit of the distillation column.
Sulfur compounds such as H2S, mercaptans, etc. are also linked to severe corrosion issues in crude oil refining units. Most of the H2S leaves with the vapor fraction of the crude oil, while some residual H2S and other sulfur compounds are present in the liquid distillates of crude oil. In the vapor line of the distillation column, ammonia (NH3) and filming amines are injected while H2 is used to remove all sulfur compounds from the liquid distillates (hydrodesulfurization). Any entrained H2S and CO2 leaving with the light ends from the top of the overhead separator is removed in the acid gas removal plant with reactive aqueous amine solutions. Hydrogenation reactions break down naphthenic acid into naphthenates and water, hence altering the corrosion reaction route.