What Does
Exchange Current Density Mean?
Exchange current is the rate of either oxidation or reduction at the equilibrium electrode. It is expressed as a current. It is also technically a misnomer, since there is no net current flowing. Exchange current represents the rates of both oxidation and reduction for a given single electrode at equilibrium.
Exchange current density can be defined as the current density that flows equally in equilibrium and in both directions. The larger the exchange current density, the faster the reaction, and vice versa. When we talk of equilibrium, it means that there is no gain or loss experienced by the electrode material. The reaction in the electrode proceeds at equal rates. This is where the reaction proceeds both in the forward and in the reverse directions to result in a zero net current and a zero net reaction rate.
Corrosionpedia Explains Exchange Current Density
When describing the corrosion of a specific reaction, the exchange current is the current from each of the two directions of the reversible reaction. These currents are denoted as "Ia" for anodic current and "Ic" for cathodic current. The reactions can be described as:
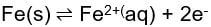 for anodic reaction and the reversed anodic reaction.
for anodic reaction and the reversed anodic reaction.
The resultant current of the above reaction is zero since the net current at equilibrium is zero. Exchange current can only be determined experimentally. In electrochemical characterization, the current value is normalized by the electrode surface area and the current density expressed as (i).
The main variables that help express the exchange current density include:
- Metal composition – Exchange current density is very dependent on the composition of the metal or alloy and on the solution. It depends on the metal that supports the redox reaction at equilibrium.
- Roughness of the metal surface – It is expressed as a geometrical surface area known to depend on the surface roughness of the metal. The higher the exchange current density, the larger the surface area.
- Concentration of soluble species – Exchange current density is also dependent on the concentration of the soluble species in that it is a complex function of both the reactants' and the products' concentrations.
- Impurities – These are impurities that dissolve on the surface of metals that tend to reduce the exchange current density. These include arsenic, sulfur and antimony.