A transition metal is one of a group of 40 metallic chemical elements in the Periodic Table. They are defined by their electronic structures incorporating d-shell electrons and they are known for their ability to exhibit and move between different oxidation states as a result. Transition metals in their pure form are generally corrosion resistant, owing to the fact that they can form stable oxide layers on the surface. They don't react readily with water and oxygen (unlike the alkali metals of the Periodic Table) but will react with oxygen at elevated temperatures. Transition metals also form alloys, which is significant in corrosion mitigation, and are ductile and malleable too.
There are four rows of transition metals in the Periodic Table. The top row ('the 3d elements') contains metallic elements that are most familiar. In order of increasing atomic mass and appearance in the table, these are: scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper and finally zinc. The whole set of transition metals can be seen in the Periodic Table below:
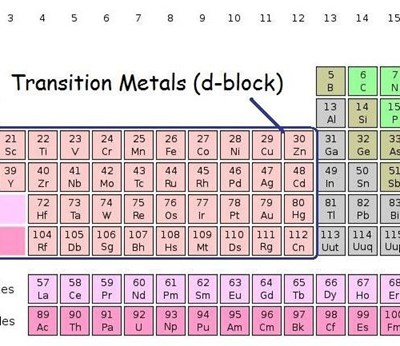
As a result of their d-shell electronic structures, the transition metals exhibit many different chemical properties including the formation of co-ordination compounds (complexes) as well as functioning as catalysts through their oxidation-reduction behavior. Transition metals also have high melting points and boiling points when compared to the softer alkali metals of the Periodic Table.
Transition metals and corrosion mitigation
IRON
Iron is arguably the most well-known transition metal for its biological necessity and as the principal element in steel, which is an alloy of iron and carbon. The natural tendency for pure metals to revert to their native (ore) oxidation state represents the continuous battle for industry against the effects of corrosion. For iron this is the conversion from Fe(0) to the iron (III) oxidation state, which is explained more here (link to corrosion page)
The incorporation of other transition metals into steel has strengthening and resistance effects that further improve the suitability of different steels for structural and non-structural applications. Many of these are reviewed below in their order in the Periodic Table:
TITANIUM
Titanium is a very strong metal known and used for its lightweight qualities; in the industrial area that includes aerospace and medical applications. Titanium does not corrode and is therefore ideally suited to exterior applications such as pipes and hand rails, bridges and tunnels. Rain and sea-water have no corrosive effects on titanium as titanium forms a passive film that prevents it from corroding.
Titanium steel does not actually contain titanium at all, but is a misnomer used for stainless steel.
VANADIUM
Vanadium is very effective at increasing the hardenability of steel, so is used in tiny amounts in steel production. Vanadium is generally used in steel alloys and has benefits of being resistant to most, but not all, dilute mineral and organic acids. The exception is that vanadium corrodes rapidly in aqueous nitric acid.
CHROMIUM
Chromium is one of the most important transition metals for steel production and most stainless steels contain about 18% chromium. Its hardness and corrosion resistance make it one of the most valuable and indispensable metals for industry. When chromium is added to steel, it increases the hardenability of steel and improves corrosion resistance in oxidizing environments. It is the element which is added to iron to create stainless steel (as opposed to just carbon, which produces mild steel).
MANGANESE
All steels contain small amounts of manganese as it is an essential requirement for the processing that converts iron into steel. Small amounts of manganese are added to iron ore in the conversion process to combine with any traces of sulphur and oxygen that are present. When manganese is added to iron, it reduces the brittleness of the steel, and adds strength.
Manganese is added to low-carbon steels to mitigate against breaking or cracking under pressure. This reinforces their strength and durability. It also increases corrosion resistance in carbon steels, thereby extending the lifetime of manufactured goods. Overall, the addition of manganese to steel delivers steel of superior performance and greater strength.
COBALT
The addition of cobalt to steel increase its hardness and heat resistance, making it ideally suited to high-temperature applications such as jet engines and gas turbines. Cobalt steel is also used in the oil and power generation industries. Cobalt steel may be considered a 'super alloy' since it possesses very high corrosion resistance, even at temperatures exceeding 1200°F.
NICKEL
Pure elemental nickel is both oxidation- and corrosion-resistant, hence it is ideally suited to corrosion-resistance applications. The noble (unreactive) nature of nickel lends itself to nickel coatings on steel, zinc and other metals to confer a host of a chemical properties, among them corrosion, erosion and abrasion resistance. Nickel coatings also help to minimize corrosion fatigue, and are mainly used for decorative purposes. Fewer than 10% of their use goes on engineering applications.
COPPER
Many properties of copper, such as its malleability, heat resistance, water resistance, corrosion resistance and natural antimicrobial properties make it the perfect choice of metal for piping both cold and hot water. Weathering steels also draw on small amounts of copper being added to steel, to increase atmospheric corrosion resistance substantially.
Copper is not always a desirable element to have in steel, but it can have corrosion resistance benefits where steel is used in saltwater or sulfuric acid environments. The presence of copper at steel surfaces draws traces of sulfur, leading to the formation of copper (I) sulfide, which helps to reduce pitting. This preferential combination with sulfur offsets the chance of more detrimental sulfur-based corrosion taking place elsewhere on the surface.
Copper alloys and nickel alloys are among the most corrosion resistant materials that can be used in engineering projects, along with aluminum alloys and stainless steel.
ZINC
Zinc has excellent corrosion resistance qualities and makes a good protective coating for iron. Where zinc does corrode, it reacts with moisture, forming zinc hydroxide which may then react with carbon dioxide to form zinc carbonate. Zinc carbonate formation results in a protective layer on the metal surface. For more on how zinc is used in anticorrosive applications, see Does Zinc Rust?
Other Transition Metals in the 4d and 5d Groups
MOLYBDENUM
Molybdenum is found in chromium-molybdenum alloy steel – sometimes called chrome moly – and this steel is ideally suited to high-temperature and high-pressure environments. Its combination of corrosion resistance, temperature resistance and strength make it attractive for use in the energy, construction, oil & gas and automotive sectors. Molybdenum provides the steel with increased strength and higher working temperatures, while the chromium content enhances oxidation- and corrosion-resistance.
TUNGSTEN
Tungsten is a supremely durable metal that is added to steel and is three times more rigid than steel. Tungsten steel does not rust except at very high temperatures (>1000°F). It is complex and expensive to produce.
Corrosion Inhibitors Containing Transition Metals
Anodic inhibitors, as part of the wider group of chemicals known as corrosion inhibitors, may contain transition metal anions such as chromate, molybdate and tungstate. These compounds form protective oxide layers on steel that passivate the steel surface and make it less vulnerable to corrosion. Other types of corrosion inhibitors do not rely on transition metals for their function.