Cooling towers provide an effective method of heat rejection and are widely used for space conditioning, refrigeration and industrial cooling applications. The control of corrosion in cooling water systems is a major challenge for many industries all over the world.
In cooling water circuits, corrosion and scaling problems are not new, but continuing trends in environmental legislation are leading to ever greater degrees of evaporation and consequently to very high residual concentrations of various species. Therefore, even if the waters used are initially clean and non-corrosive, because of this concentration effect, they become corrosive and their tendency to induce scaling and biofouling increases.
In this article, we'll take a look at cooling towers, the corrosion issues they most commonly face, and how to prevent them.
Types of Cooling Tower Circuits
In a closed circuit, all the cooling water is confined in a closed loop. There is no contact with the atmosphere and therefore no risk of contamination by the latter. Heat is removed by conduction and convection via a secondary circuit and not directly by evaporation of the primary circuit water. Closed circuits can only be used in small-sized plants, in high-flow-rate systems, or in systems with a refrigeration unit (iced water tank).
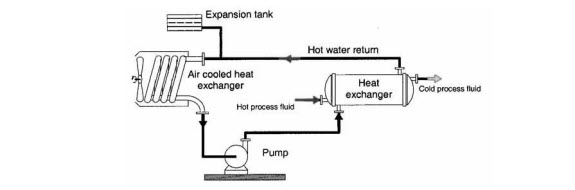 Figure 1: Close Recirculating Cooling System
Figure 1: Close Recirculating Cooling System
Source: Control of Corrosion in Cooling Waters, edited by J.D. Harston and F. Ropital
Open recirculating cooling systems, as shown in Figure 2, are the most widely used. The semi-closed circuit is fed by a feed-water supply. The circulating water flow rate is maintained constant by pumps. The water is heated by the hot process fluid in the heat exchangers. The hot water is in direct contact with the air in the cooling towers, and is cooled both by this contact and by loss of latent heat of evaporation.
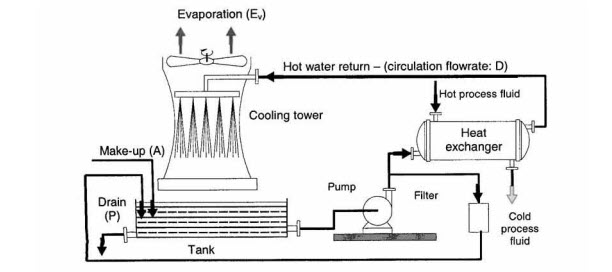 Figure 2: Open Recirculating Cooling System
Figure 2: Open Recirculating Cooling System
Source: Control of Corrosion in Cooling Waters, edited by J.D. Harston and F. Ropital
Problems Arising from the Use of Untreated Water
The three major types of problems in cooling water circuits are scaling, corrosion, and fouling. These problems are strongly interrelated and corrective actions taken to treat one of them frequently have repercussions on the others.
Scaling
Scaling is considered to occur when a metallic or other surface becomes covered by an adherent mineral deposit. The distinguishing feature compared to a deposit produced by the sedimentation of solid particles from the liquid is the fact that the scale adheres to the surface. Scale deposits can enhance trapping of suspended solids.
In a water-fed cooling circuit, scaling is essentially due to the formation of calcium carbonate. The scale may subsequently contain other substances, such as clays, algae residues, or calcium sulphate, but it is always calcium carbonate that precipitates first, since its solubility is lower.
Corrosion
Aqueous corrosion of metals is electrochemical in nature and involves two independent reactions. The first reaction corresponds to oxidation of the metal. The second reaction is a reduction of some species in the corrosive medium. The metal oxidation reaction is anodic and releases positively charged metal ions into the solution and electrons into the metal as shown in the below formula:
(M)metal –> (Mn+)solution + ne–
Subsequently, the electrons liberated in the metal reduce an oxidant in the corrosive medium in the cathodic reaction described below:
(Ox+q)solution + (ne–)metal –> (Redq-n)solution
A wide variety of corrosion modes can occur depending on the medium and materials concerned. For steel alloys, the most common corrosion modes are uniform corrosion, pitting corrosion, crevice corrosion and intergranular corrosion. For copper alloys, corrosion modes can include dezincification and Al or Ni depletion.
Corrosion Fouling Induced by Micro-Organisms
Micro-organisms are present naturally in all waters. If they proliferate too rapidly they can create two types of problem in water circuits:
- Biofouling, which relates to the accumulation of micro-organism colonies on equipment surfaces, leading to the formation of biofilms.
- Biocorrosion, which relates to chemical attack by micro-organisms. In the case of metals, the corrosion is generally due to bacteria.
In both cases, the consequences of the proliferation of micro-organisms can be important, with loss of efficiency of heat exchangers, obstruction of piping, increased back pressures and even leakage by breakthrough corrosion.
Treatment of Water Circuits
The purpose of the upstream feed-water treatments is to modify the properties of the raw water to meet the requirements of the circuit concerned. Regardless of the treatment of the feed-water, it is still necessary to add chemicals to the water in the cooling circuit. This is because a specific site conditioning is required to ensure the success of the treatment philosophy adopted. The common chemical products are scale inhibitors and dispersants, corrosion inhibitors, and biocides.
Scale Inhibition and/or Dispersant Treatments – Stabilization
In this process, additives are injected into the circuit to prevent the precipitation of calcium carbonate, particularly at hot points. These products either increase the solubility limit or maintain the water in a state of super-saturation. They thus enable the circuit to operate at a higher concentration ratio.
The major mechanisms involved are:
- Sequestration/complexing to form stable molecules with calcium and magnesium ions
- Poisoning of crystal nuclei
- Inhibition of crystal growth
- A dispersion effect to maintain the nucleating particles in a state of dispersion close to their solubility limit.
Stabilization treatments are very popular, since they allow operation at "free pH."
The pH is then controlled by the CO, and solubility equilibrium between the water and the atmosphere, and becomes a simple function of the M-alkalinity level (MA).
pH control
The solubility limit of CaCO3 is sensitive to the pH, which directly affects the concentration of carbonate ions. In order to prevent CaCO3 precipitation, acid is injected into the circuit to lower the pH. Sulphuric acid is usually chosen for this purpose.
In fact, the addition of acid has two effects: it decreases the MA level by neutralizing HCO3- ions, forming CO2. It also lowers the pH if the CO2 is generated more rapidly than it is removed from the circuit by degassing.
Corrosion Inhibition Treatments
When corrosion inhibitor is added to the circuit, the product will form thin adsorbed films that do not hinder the heat transfer. They contain two active agents in order to impede both the anodic and cathodic corrosion reactions.
Anodic Inhibitors
Anodic inhibitors increase the anodic polarization and move the corrosion potential to the cathodic direction. These substances combine with the metal corrosion products to form a completely insoluble salt. If inhibition is purely anodic, large quantities of inhibitor are necessary. This can only be practical in very small volume circuits, since any inhibitor deficiency can lead to accelerated localized attack.
Cathodic Inhibitors
Cathodic inhibitors reduce corrosion by slowing the reduction reaction rate of the electrochemical corrosion cell. These substances combine with the products of the cathodic corrosion reaction to again form insoluble compounds. Cathodic inhibitors involve lower risks than their anodic counterparts, since localized corrosion is not induced by a fall in their concentration.
Organic Inhibitors
The effect of organic inhibitors is related to the formation of a continuous adsorbed film, which hinders electrochemical reactions at exposed surfaces. The film is formed by the physical or chemical adsorption of polar organic molecules on the metal surface, so that the choice of molecules depends on the metal concerned.
Implementation of Treatment
After the conditioning treatment has been defined, it is then implemented in accordance with the specific features of the site. For closed circuits, the conditioning products are injected on startup. No further additions are necessary except in the event of accidental draining.
In open recirculating water systems, the reagents, except for non-oxidizing biocides, are injected periodically via pumps in the feed reservoir. In rare cases, gravity injection is employed. Additional point treatments may sometimes be necessary in certain critical heat exchangers.
In more recent years, real-time corrosion monitoring systems are employed to assess the general corrosion rate as well as evaluate the potential for localized, or pitting, corrosion. These systems use a combination of technologies, including electrochemical noise (ECN), low-frequency impedance (LFI) and harmonic distortion analysis (HDA).