I have often been asked this question: “Which testing method do you prefer to use to check an abrasive blasted steel surface profile for coating: Testex replica tape, ISO comparator or profilometer?” (For a discussion of replica tape, read Replica Tape: A Source of New Surface Profile Information.)
To be honest, I trust my sense of touch more than any of these methods. This answer was once echoed by a former colleague who worked in the industry for more than 30 years. He further commented: “In the past, when I found my finger was bleeding after touching freshly blasted steel, I knew I had a good surface profile. But today, with all these non-bloody measurement tools and coating specifications, the surface preparation problem in the industry has not been reduced but rather it occurs even more.” (For more on abrasive blasting, see Wet Abrasive Blasting: An Overview of Surface Cleaning Alternatives.)
Is There Room for Improvement?
This opinion reflects what may be a common experience in the coating industry. Surface preparation amounts to 90% of the work on a coating project, but even when performed to specification, it still contributes to coating failures.
Are our current coating standards and specifications adequate to help us define the proper requirement to ensure that a coating meets its design life?
Here is an example of a typical coating specification: “The steel surface shall be blast cleaned to a near-white NACE No. 2/SSPC-SP10 or Sa2.5 finish, with an angular surface profile height minimum of 2.5 mils (63 microns), or between 1.5 mils and 4.0 mils (38 microns and 100 microns).”
Similar surface profile requirements are used in most internationally accepted coating standards and project specifications, including API, ISO and SSPC.
But a specific range of surface roughness is not the true deciding factor. In fact, the intensity of a coating's adhesion to an abrasive-blasted substrate does not depend on any length parameter of surface roughness, regardless of whether the roughness parameter is a profile linear height of Ra, Ry and Rz, or peak count (peaks per linear length).
Instead, the critical question is whether a roughened surface has a higher surface energy and lower contact angle in order to provide adequate wettability for the coating. Let me explain.
The Science of Adhesion
Let’s begin with the end in mind. The reason for surface preparation by abrasive blasting is to create a clean substrate surface to which a coating can adhere, in order to achieve the required bond strength (adhesion) of the coating during its designed service life.
Therefore, before we further discuss the measurements and specification requirements for a proper surface profile of an abrasive blasted surface, we will first need to go back to the basic science of adhesion, which might not be well understood by many corrosion engineers in the industry.
Point One: Adhesion Mechanisms
First, adhesion mechanisms can be divided into three basic types:
- Mechanical interaction
- Thermodynamic mechanism
- Chemical bonding
Among these, mechanical interaction contributes significantly. Mechanical adhesion to an abrasive blasted substrate relies on the curing (hardening) of the coating inside the surface profile and asperities of the substrate surface and physical anchorage resulting therefrom.
The mechanical bond may be assisted by contact friction between the substrate and coating in areas where the actual adhesion is inadequate.
Principle: Mechanical adhesion in tension differs significantly from mechanical adhesion in shear. For example, a high interface roughness may improve shear bond strength, whereas tensile mechanical bond strength primarily depends on vertical anchorage in the surface profile.1
Second, adhesion is also defined as a process through which two bodies come into contact and attach (bond) to one another in a way that requires external force or thermal motion to break the bond. Therefore, adhesion has two different aspects:
- In the conditions and the kinetics of contact
- In the separation process
The intensity of adhesion will depend not only on the energy that is used to create the contact, but also on the interaction existing in the contact interface.
In the case of contact between a liquid coating and a solid substrate, this contact process is called wetting. The ability to wet a surface can be defined as the propensity of liquid to spread on a solid surface. A liquid will wet a solid when its surface energy is lower than the solid’s surface energy.
Principle: Liquid deposited on the solid surface under gravity has a tendency to spread until the cohesion (internal forces) of the liquid, the gravity forces and the capillary (surface tension) forces are in balance, and an equilibrium state is reached.
Force balance or equilibrium at the solid-liquid boundary is given by Young’s equation for contact angles greater than zero (see Figure 1):2

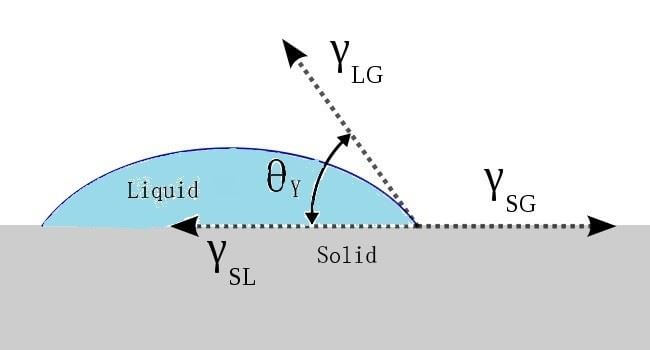
Figure 1. Contact angle of a liquid droplet wetted to a rigid solid surface.
The lower the contact angle, 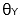 , the greater the tendency for the liquid to wet the solid, until complete wetting occurs (contact angle
, the greater the tendency for the liquid to wet the solid, until complete wetting occurs (contact angle  . For complete wetting to occur, the surface tension of the liquid should be less than, or equal to, the critical surface tension of the substrate (YSG – YSL). Large contact angles are associated with poor wettability. The Young equation assumes that a surface is ideal when it is both chemically homogenous and topographically smooth.
. For complete wetting to occur, the surface tension of the liquid should be less than, or equal to, the critical surface tension of the substrate (YSG – YSL). Large contact angles are associated with poor wettability. The Young equation assumes that a surface is ideal when it is both chemically homogenous and topographically smooth.
However, this is not true in the case of a roughened surface (such as abrasive blasted), which instead of having one equilibrium contact angle value, it exhibits a range of contact angles between the advancing and receding ones.
The relationship between the roughness of a surface and wettability of a liquid was defined in 1936 by Wenzel, who stated that adding surface roughness will enhance the wettability caused by the chemistry of the surface. Wenzel's statement can be described with the equation:3

The roughness ratio is defined as the ratio between the actual and projected solid surface area (r=1 for a smooth surface, and >1 for a rough one). For a rough surface, the roughness ratio (r) can be calculated from a 3-D roughness parameter (Sdr), which is the developed surface area ratio, representing the additional surface area contributed by the rough texture:

Wenzel’s theory has provided a base for a common claim in the industry regarding surface preparation for coating/adhesive application:
Increasing the roughness of a surface would improve the adhesion of a liquid coating or adhesive to it.
However, the Wenzel equation is based on the assumption that a rough surface extends the solid-liquid interface area in comparison to the projected smooth surface. As a result, the liquid has penetrated and then covered completely into the roughness grooves.
This implies that Wenzel’s simple model is only useful in capturing the situation of simple roughness topography, and for well-wetting surfaces where the practical range of the contact angle, 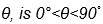 .
.
For coating purposes, it is important to note that in cases where the liquid has any difficulty penetrating into the surface grooves to fully cover them, and where gas molecules (such as air) can be trapped in the asperity valleys, the Wenzel equation does not apply.
Principle: On a rough surface where the interface between liquid and solid is not continuous and there is an alternation of solid-liquid and gas-liquid interfaces, the geometry of the roughness has to be carefully designed or determined in order to reveal the true relationship between roughness and wetting.
Point Three: Capillary Force
Thirdly, in addition to gravity force, capillary force due to surface tension is the main driving force for the wetting process—for a liquid to spread on a rough solid surface. Capillary adhesion occurs when two wetted surfaces are brought into contact. When the contact angle is smaller than 90°, the surfaces stick together strongly. Between grains of sand, for example, water forms bridges that are partially responsible for the stability of sandcastles.
Surface energy is sensitive to three surface conditions:
- Chemistry of the surface
- Morphology
- The presence of adsorbed materials
For this reason, the adsorption of surface chemicals lowers its surface free energy (wettability). Therefore, surfaces with high surface energies will have a strong tendency to adsorb materials (e.g. moisture or dust particles) from the atmosphere, thus reducing wettability.
Surface active agents, or surfactants, can alter the properties of wetting and capillary action. On a rough surface, capillary absorption of the liquid plays an important role in the anchorage effect, because it draws material paste into peaks and valleys of the substrate.
A good understating of the above adhesion theory is very important when we apply it to review the measurement and specification requirements of a surface profile of an abrasive blasted surface to accept a coating for long-term corrosion protection.
A Better Way to Measure Surface Profiles
In Questioning Current Methods in Defining Proper Surface Profile (Part 2), we will put this science into practice in considering a better way to measure and define a properly blasted steel surface profile to achieve good coating adhesion.
***
References:
- H.D. Beushausen, “Long-Term Performance of Bonded Overlays Subjected to Differential Shrinkage,” PhD Thesis, University of Cape Town, South Africa, 2005, 264 pp.
- T. S. Chow, Wetting of rough surfaces, J. Physics: Condensed Matter 10 (27): L445, 1998.
- R.N. Wenzel, Ind. Eng. Chem. 28, p988, 1936.