Metal dusting is a catastrophic form of corrosion degradation. It occurs when susceptible metals and alloys disintegrate rapidly into a dust of fine metal particles and graphitic carbon. The metal dusting phenomenon has been observed in austenitic stainless steels, ferritic steels and various nickel (Ni)-based materials in petrochemical and chemical plants while hydrocarbons or other high-carbon-activity atmospheres are processed at an elevated temperature of 400 to 900 degrees Celsius.
Thermodynamic Considerations
The carbon source catalyzing metal dusting can be a hydrocarbon or carbon monoxide (CO). And, when it happens, carbon from the high-carbon-activity atmosphere may either be dissolved, form carbides or be deposited on the metal surface as graphite. The thermodynamic carbon activity determines which of the aforementioned outcomes occurs.
Thermodynamic carbon activity is the equilibrium effective concentration of carbon in a process stream. For a hydrocarbon-hydrogen mixture, you can calculate the carbon activity for the reaction CxHy = y/2 H2 + xC at equilibrium using the following formula:
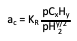
In this equation, "p" is the partial pressure of the component in the gas phase; and "KR" is the equilibrium constant. For CO reduction, the carbon activity per the reaction 2CO = CO2 + C at equilibrium would be:
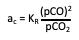
When the Carbon Activity is Less Than One
In this case, carburization will occur—with the carbon deposition in high temperature alloys leading to internal carbide formation of M7C3 and M23C6, which then converts to M7C6. Carburized metals can fail at temperatures above about 1000 degrees Celsius from internal cracking—which, itself, is a result of the volume increase from internal carbide formation. (For more on this topic, see: Chloride, Caustic and Polythionic Acid Stress Corrosion Cracking.)
Carburization of metals at lower temperatures, when strained at room temperature, can fail because of the carburized metal's lower ductility/toughness.
Carburization of ethylene steam cracking tubes is a common failure mechanism. For that reason, some petrochemical organizations will specify metallurgy replacement—such as heater or reactor tubes—when the carburized metal layer has grown to a specific fraction of the wall thickness. They may also require shutdown conditions that minimize thermal shock.
When the Carbon Activity is Greater Than One
Here, graphite will form on the metal surface.
A carbon activity of around one will produce metal catalyzed or filamentous coke. Such coke can grow rapidly and plug a reactor.
A carbon activity much greater than one will result in metal dusting and rapid metal loss. Metal dusting from gases containing CO and hydrogen (H2) from the conversion of methane used to synthesize methanol (CH3OH) or ammonia (NH3) can have very high carbon activities.
In general, Ni-based alloys are considered more resistant to metal dusting corrosion than iron (Fe)-based alloys. That's because the reaction path for Fe-based alloys and Ni-based alloys is different.
For Fe-based alloys, such as austenitic stainless steel, the carbon initially ingresses into the metal phase and oversaturates the metal. Cementite (Fe3C) forms—the process becoming a barrier to further carbon ingress. This causes graphite precipitation—with the Fe3C becoming unstable and decomposing to C + 3Fe. The carbon atoms from this decomposition attach to the basal planes of graphite, which grow into Fe3C. The metal atoms then diffuse through the graphite and agglomerate as nano-sized metal particles. (A different proposed mechanism suggests the Fe3C does not decompose with the nano-sized Fe3C particles moving away from the metal surface. Rather, it claims these particles act, for example, as catalysts by dehydrogenating the hydrocarbon and producing additional coke growth.)
On the other hand, for Ni-based alloys—such as Alloy 600—the mechanism differs because of the absence of a metastable carbide. Instead, carbon continuously ingresses into the metal. The carbon becomes oversaturated at the surface, resulting in graphite formation on the surface and into the metal. Metal destruction results from the formation of nano-sized metal particles that catalyze graphite growth.
The observation of metal dusting includes significant amounts of graphite and deep pitting. Upon cross-section, you may notice a surface layer of carburization. The graphite is filamentous; and you'll often note metal catalyzed with metal particles at the tips of the filaments. Coke can grow between the filaments making it dense and difficult to observe the filaments by scanning electron microscopy. (For more on this topic, see: Understanding Pitting Corrosion to Prevent Catastrophic Failures.)
Disrupting any of the mechanism steps can slow metal dusting down. Carbon and low-alloy steels have low carbon solubility and high carbon diffusivity because of their body-centered cubic crystal structure. Thus, they are highly susceptible to carbide formation. Austenitic alloys, such as stainless steel and Ni-based alloys, have face-centered cubic crystal structures and thus low carbon diffusivity and high carbon solubility. This results in a lower tendency for carbide formation. Since carbon diffusivity decreases with Ni content, Ni-based alloys, in general, have better resistance to metal dusting corrosion than austenitic stainless steels.
Oxide Scales
Oxide scales can play an important role in reducing alloys' susceptibility to metal dusting corrosion because an oxide layer can slow the diffusion of carbon into the metal. Chromium oxide (Cr2O3) is one of the most potent oxide scales. Maintaining the continuous oxide protective scale is critical for mitigating the metal dusting corrosion. At elevated temperature under reducing conditions such scale may not be maintained. Moreover, defects or oxide compositional changes—such as spinels (Fe1+xCr2–xO4)—might be produced, which may not be protective. Addition of water or oxygen (O2) may be required to maintain the oxide film.
Calculation of Phase Diagrams (CALPHAD) thermodynamic calculations can help you evaluate necessary amount of chromium in the alloy and whether the protective oxide film can be maintained for the specified process conditions.
Other oxides, in combination with Cr2O3, can provide metal dusting resistance. The most effective alloying additions to achieve this are aluminum (Al) and silicon (Si). Typically, the outer continuous scale is rich in Cr2O3 and the inner continuous scale is rich in Al2O3 or SiO2. Thus, Alloys 601 and 602—which have Al and higher Cr—have greater resistance to metal dusting corrosion than Alloy 600.
When the protective oxide scale becomes defective, adding low levels of hydrogen sulfide (H2S) to the process stream can minimize carbon ingress by slowing the carbon transfer from the atmosphere. The adsorbed sulfur can also suppress graphite nucleation and inhibit graphite growth. However, the sulfur must usually be removed from the product stream as it can potentially interfere with the desired process reactions. Neutralization may be required to prevent polythionic acid stress corrosion cracking of austenitic stainless steel if a sulfide scale is formed. (For more on this topic, see: Polythionic Acid Stress Corrosion Cracking of Austenitic Stainless Steel.)
Non-Porous Barrier Coatings
To reduce metal dusting corrosion, an impervious, non-porous barrier coating can be applied to the metal surface. Some coatings, such as those applied by chemical vapor deposition, fall victim to the following caveats:
- They have limitations on the size of the component to be coated.
- They have issues with field repairs.
- They require special welding procedures.
- They may have a thermal expansion coefficient mismatched with the base metal.
To overcome the last point in the above list, you can apply multiple diffusion barrier coatings to the base metal with a top passivation coating of, for example, alumina.
New Metal Dusting-Resistant Alloys
New alloys have been developed that claim to be resistant to metal dusting corrosion. These alloys tend to:
- Be high in Cr, Al and Si.
- Be low in Fe.
- Sometimes, have copper (Cu).
- Have a controlled grain size.
However, more extensive testing is required before these alloys become widely specified.
Chromium
A high amount of chromium is important in any stainless steel and in nickel alloys for metal dusting corrosion resistance, as this ensures a stable passive layer of chromium oxide. A latest alloy, VDM Alloy 699XA, has a maximum chromium content of 30%; and because of that it is one of the better alloys to use in this application.
However, any localized rupture of the chromium oxide layer can result in significant carbon intake from the process atmosphere into the alloy. Here, the addition of aluminium causes the formation of a protective aluminium oxide scale or sub-scale. F. VDM Alloy 699XA has an aluminium content as high as 2%. A much higher aluminium content, such as 3%, would reduce workability.
Increasing chromium to about 30%, together with a low iron content, is necessary for high metal dusting corrosion resistance in nickel alloys. (For more on this topic, see:
All About Environmental Cracking in Nickel-Based Alloys.)
This is important because heat-resistant stainless steels and nickel-based alloys are commonly used for furnace inner parts and heat treatment fixtures in the heat-treating industry. These components are often replaced due to a variety of factors. By understanding some of the more common causes for failure—particularly corrosion—it is possible to extend these components' lives through things like improved design and material selection.
Conclusion
Metal dusting, also known as catastrophic carburization or carbon rot, is a metal wastage and it is not an embrittlement phenomenon. In the right environment (i.e., one that is carbon-rich with temperatures around 1100 degrees Fahrenheit), any alloy can eventually have metal dust.
Regarding appropriate alloy selection, there is no common agreement. Generally, nickel alloys with high chromium contents and additions of silicon and/or alumina provide improved performance. In the steel heat-treating industry, based on experience, RA333 and Supertherm are two of the best alloys for resistance.