What Does
Eutectic Reaction Mean?
A eutectic reaction is a special type of phase reaction – a three-phase reaction process in which a liquid solution that is cooled to the eutectic temperature results in two solid phases occurring at the same time. For example, a liquid alloy becomes a solid mixture of alpha and beta at a specific temperature, rather than existing over a temperature range.
The eutectic solid is commonly lamellar (stripy) in form.
Corrosionpedia Explains Eutectic Reaction
A eutectic reaction is expressed as follows:
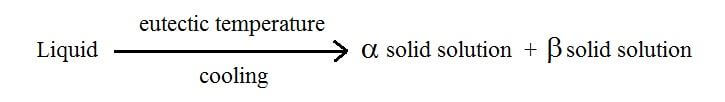
The eutectic reaction is an invariant reaction since it occurs in the complete thermal equilibrium state. This means that upon cooling the liquid solution under eutectic temperature results into two solid solutions, both coexisting at the same time and in chemical equilibrium.
Eutectic properties are exhibited in certain types of alloys and therefore these are also known as eutectic alloys. These alloys are formed from two or more compounds that have eutectic compositions. These alloys possess austenite alloy properties and are highly corrosion resistant, have high compressive strength and have a glassy appearance. These alloys can be used in factories to manufacture industrial equipment or used as alloy formula for soldering in electronics and nuclear reactors.