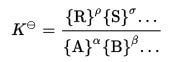What Does
Equilibrium Constant Mean?
The equilibrium constant is the ratio of the concentration of products to the concentration of reactants. In any chemical reaction, the equilibrium constant is obtained when the chemical reaction reaches equilibrium. Once this is achieved, the concentration of every molecule involved in the reaction is measured. Since the chemical reaction took place in an equilibrium state, the equilibrium constant does not change for a given reaction.
The equilibrium constant is one of the essential parameters in understanding various chemical systems and biochemical processes.
Corrosionpedia Explains Equilibrium Constant
In a chemical reaction, the equilibrium constant is the value of the reaction quotient when the reaction has reached the equilibrium. The equilibrium constant does not depend on any analytical concentration of any product and reactant in a mixture, but does depend on the ionic strength of the reactants and products and the temperature at which the solution is kept when a chemical reaction takes place.
Following are two important points to consider:
- The equation of the chemical reaction should be balanced, only then will the system be in equilibrium
- The pressure and concentration of the reactants and products in a solution should also remain in equilibrium
Once the above points are considered, the equilibrium constant is calculated after substituting values in the equilibrium expression below: