What Does
Calorie Mean?
A calorie is amount of heat necessary to increase the temperature of one gram of water by 33.8°F (1°C). Necessary heat depends on two variables: atmospheric pressure and starting temperature. The value of one calorie is equal to 4.1860 joules.
Both calories or joules are used in calculating change of Gibbs free energy. They can also be used in calculating electromotive force (EMF) of a cell and half-cell potential.
Corrosionpedia Explains Calorie
One calorie is the heat required to raise the temperature of 1 gram of water by 1°C at a constant atmospheric pressure. The pressure is the standard atmospheric pressure (101.325 kPa).
The Gibbs free energy change,  is the measure of tendency for any chemical reaction to happen. It includes the reaction of a metal with its environment. When the value of
is the measure of tendency for any chemical reaction to happen. It includes the reaction of a metal with its environment. When the value of 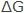 is more negative, a reaction has a greater tendency to occur.
is more negative, a reaction has a greater tendency to occur.
Considering electrochemical mechanisms of corrosion, the potential for a metal to corrode can also be expressed in terms of the electromotive force (EMF) of the corrosion cells. It is an integral part of the corrosion process. Since electrical energy can be expressed in joules, the relation between 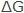 in joules and EMF in volts (E) can be shown by
in joules and EMF in volts (E) can be shown by 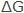 = – nFE , where n is the number of electrons taking part in the reaction, and F is the Faraday (96,500 C/eq). Therefore, the term
= – nFE , where n is the number of electrons taking part in the reaction, and F is the Faraday (96,500 C/eq). Therefore, the term 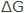 can be converted from calories to joules by using the value 1 cal = 4.184 absolute joules. The greater the value of E for any cell, the tendency for the overall reaction of the cell to occur is higher. This means that the higher the value of E, the higher the possibility of corrosion.
can be converted from calories to joules by using the value 1 cal = 4.184 absolute joules. The greater the value of E for any cell, the tendency for the overall reaction of the cell to occur is higher. This means that the higher the value of E, the higher the possibility of corrosion.