What Does
Catalytic Hydrogenation Mean?
Catalytic hydrogenation is treatment with hydrogen in the presence of a catalyst such as nickel, palladium or platinum. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
Catalytic hydrogenation has diverse industrial uses. Most frequently, industrial hydrogenation relies on heterogeneous catalysts. In petrochemical processes, hydrogenation is used to convert alkenes and aromatics into saturated alkanes (paraffins) and cycloalkanes (naphthenes), which are less toxic and less reactive as well as less corrosive. Hydrocracking of heavy residues into diesel is another application.
Corrosionpedia Explains Catalytic Hydrogenation
Catalytic hydrogenation is hydrogenation in presence of catalysts. Addition of hydrogen to alkenes is an exothermic (releasing heat energy) reaction, requiring the use of a transition metal catalyst due to the high energy barriers to direct the reaction between alkenes and hydrogen gas. Catalytic hydrogenation of alkynes with transition metal catalysts results in alkanes (just like the catalytic hydrogenation of alkenes) unless specially deactivated catalysts are used.
For the petrochemical industry, many of the compounds found in crude oil are of little use since they contain multiple double bonds; they must be first converted to saturated compounds before use as commodities such as gasoline. Hydrogenation is also used in coal processing. Solid coal is converted to a liquid through the addition of hydrogen. Liquefying coal makes it available to be used as fuel.
Hydrogenation reactions use a catalyst such as palladium, platinum, rhodium, ruthenium or Raney nickel, and are performed at elevated temperature and pressure.
Catalytic hydrogenation occurs on the surface of the metal catalyst. Transition metals adsorb hydrogen molecules and pi systems. The pi system (alkene or alkyne) adds hydrogen atoms in a stepwise manner as shown in below:
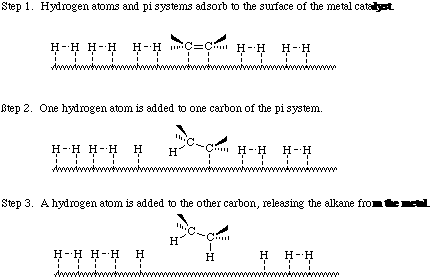
Catalytic hydrogenation has been used to reduce oxygen concentrations in seawater to a negligible level, lower than the oxygen corrosion-pitting potential.