What Does
Working Electrode Mean?
In corrosion testing, the working electrode is a sample of the corroding metal. Generally, it is not the actual metal structure being studied. Instead a small sample is used to represent the structure. The working electrode can be bare metal or coated. The working electrode can be referred to as either cathodic or anodic.
Corrosionpedia Explains Working Electrode
The Function of a Working Electrode:
A fixed potential difference is applied between the working electrode and the reference electrode. This potential drives the electrochemical reaction at the working electrode's surface. The current produced from the electrochemical reaction at the working electrode is balanced by a current flowing in the opposite direction at the counter electrode. The reference electrode acts as a reference point for the redox couple. The current resulting from the electrochemical reaction is amplified and, when plotted as a function of time, appears as a peak on the recording device.
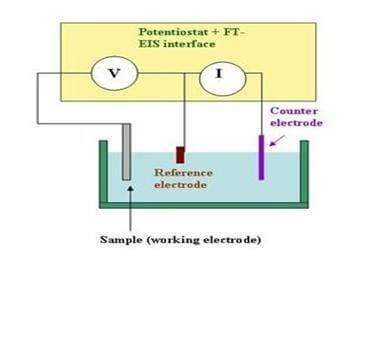
Selection of Working Electrode:
The selection of a working electrode material is critical to experimental success. Several important factors should be considered. Firstly, the material should exhibit favorable redox behavior with the analyte, ideally fast, reproducible electron transfer without electrode fouling. Secondly, the potential window over which the electrode performs in a given electrolyte solution should be as wide as possible to allow for the greatest degree of analyte characterization. Additional considerations include the cost of the material, its ability to be machined or formed into useful geometries, the ease of surface renewal following a measurement and toxicity.
Types of Working Electrodes:
A wide variety of working electrodes are available for use. The most common working electrode materials utilize carbon. Originally the carbon paste electrode was developed, but this was soon replaced by more convenient and stable carbon-based working electrodes including those made from glassy carbon, pyrolytic carbon and porous graphite. Metals such as platinum, gold, silver, nickel, mercury, gold amalgam and a variety of alloys are now also commonly used as working electrode materials.