Problems with bridges and similar infrastructure in the USA have largely concerned degradation due to old age. However, one new bridge has been the subject of much discussion because of expensive and high profile failures during construction. A number of critical anchor rods in California's $US 6.5 billion replacement San Francisco–Oakland Bay Bridge, built to last 150 years, fractured in March, 2013. As a result, the California Department of Transport (Caltrans) had to install a steel saddle retrofit to provide the required strength at a cost of $US 25 million. In addition, the department has suffered bad press and spent significant sums of money on investigations and strategies to prevent further failures.
This failure provides a good case study showing some of the technical issues and problems that can arise in modern steel and concrete structures. The fact that the failure has been played out in public, with most of the documentation freely available on the internet, has made it easy to identify the problems and follow the discussion and arguments by various parties. However, it should be noted that investigations are ongoing, and it is possible that new findings will change some conclusions and recommendations.
Construction of the San Francisco–Oakland Bay Bridge and Early Failures
The bridge uses shear keys between the deck and supporting pier to minimize bridge damage in the event of an earthquake. Large steel rods anchor these blocks to the pier. In March 2013, soon after workers tensioned these rods, a number of them fractured in two. As the roadway had been installed above the shear keys and the rods had been dead-ended, they could not be removed.
The anchor rods were 75 mm in diameter of two lengths, approximately 3 or 5.5 meters (9.8 or 18 feet) long, with both ends threaded. They were manufactured in 2008 from 4140 low alloy steel to meet the requirements of ASTM A354 Grade BD and hot dip galvanized (HDG) for corrosion protection. They were installed in the anchor holes but left to sit due to delays in the project. They were finally tensioned five years later to 75% of the ultimate tensile strength and over the following two weeks, 32 of the 96 rods (5 of the 3-meter, 27 of the 5.5-meter) fractured. The tension on the unbroken rods was reduced to prevent further failures and none have fractured since.
A metallurgical investigation was immediately carried out which concluded that failure was due to hydrogen embrittlement, although the source of the hydrogen was not identified. (Learn about this failure type in An Introduction to Hydrogen Embrittlement.) A subsequent investigation that reported in September 2014 found the hydrogen was environmentally induced as the rods had been sitting in water that had entered the pipe sleeve assemblies enclosing the rods because of the delay before grouting and tensioning. Corrosion of the zinc generated the hydrogen causing the embrittlement.
The following discussion looks at the rod material, galvanizing, grouting and other factors that have contributed to the failure and some suggestions to minimize the risk of such occurrences in the future.
Rod Material
The high tensile steel rods were manufactured, heat treated and delivered in 2008. Specification requirements, along with the results of quality control tests and tests carried out during failure analysis on the rods are given in Table 1, which show that the material easily exceeded these requirements, although some samples showed elongation at or slightly below the ductility requirements.
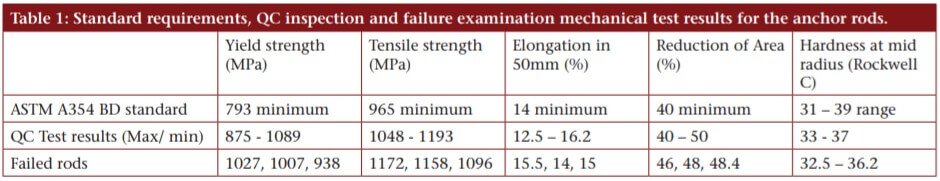
ASTM A354 Grade BD requires hardness readings at the mid radius, whereas it is the hardness at the steel surface (or just below) which is critical for development of hydrogen embrittlement. QC reports by the heat treater showed many of the rods had a surface hardness of Rockwell C 39. (Hardness testing is discussed in 5 Ways to Measure the Hardness of a Material.) Considering the relationship of this parameter to hydrogen embrittlement, this should have been investigated further.
The likelihood of failure due to hydrogen embrittlement depends on a combination of three factors: a susceptible material, a source of hydrogen and a tensile stress (applied or residual). High strength steel was essential given the loads and is a susceptible material. Caltrans disallowed pickling before galvanizing (see below) so were aware of the risk of internal hydrogen embrittlement if not embrittlement from cathodic corrosion reactions.
The risk of embrittlement increases as steel strength increases. Maximum strength or hardness limits to avoid hydrogen embrittlement have been provided in numerous sources and standards, with a typical hardness quoted of Rockwell C 35, but some sources have figures as low as Rockwell C 31. There is no definite figure as the likelihood of embrittlement depends on hydrogen content as well as strength for a susceptible steel, so a steel exposed to high levels of hydrogen will have a lower strength or hardness limit than one exposed to low hydrogen levels. The maximum Rockwell C hardness of 39 given by ASTM A354 would be considered as excessive by most sources where there is a risk of hydrogen embrittlement such as for HDG product. A more conservative upper limit on the steel hardness should have been applied. This would have not caused problems, as a slightly higher tempering temperature would have reduced hardness and the risk of hydrogen embrittlement while still meeting minimum strength requirements. As a bonus, it would have made the elongation less marginal and improved impact properties although these are not directly related to hydrogen embrittlement.

Hot Dip Galvanizing
Hot dip galvanizing is problematic with high strength steels because of the known risk of hydrogen embrittlement. It is the pickling stage rather than the galvanizing that can introduce hydrogen. ASTM A354 does not forbid HDG for grade BD, but does note that “Research conducted on bolts of similar material and manufacture indicates that hydrogen-stress cracking or stress cracking corrosion may occur on hot-dip galvanized Grade BD bolts.” However, standards for similar strength fasteners such as ASTM A490, along with proprietary post-tensioning bar “Macalloy 1030”, forbid HDG whether pickled or not. Other standards or guidelines put a limit, such as HDG is only permitted for tensile strengths below 1100 MPa or below Rockwell C hardness of 33.
Caltrans was aware of the issue of hydrogen embrittlement from HDG and specifically required rods to be abrasively blasted rather than pickled. However, this only avoids the problem of hydrogen ingress during the fabrication (sometimes called internal hydrogen embrittlement or IHE). Zinc coated items can generate hydrogen during service when exposed to corrosive environments (environmental hydrogen embrittlement or EHE) which in fact is more likely to cause embrittlement, as was the case here.
Given the potential and actual problems with galvanizing, zinc metal coating applied by any method should be avoided if possible for fasteners of such high strength in such environments. The galvanizing is designed to protect against corrosion from salt, oxygen and water that may penetrate to the bare surface although the risk is low. However, there is a very real of risk of hydrogen gas and resulting embrittlement as a result of reaction of zinc with water leaching alkali from cementitious material encapsulating the bars, especially given the planned 150 years life. This risk would be much greater than the risk of loss of section from corrosion. Uncoated steel passivates in an alkaline cementitious environment with a very low corrosion rate. Moreover, there are organic and other coatings available that would not generate hydrogen in the cementitious environment.
Grouting of the Rods
Problems with the anchor rods were further compounded when they were installed in the anchor holes. Water from rain and washing activities had entered the anchor holes and built up around the lower nut and thread. This water was analyzed and found to have a pH of 13. Clearly, the water reacted with the cement to produce a highly alkaline solution. Such conditions lead to rapid zinc corrosion, with hydrogen gas being formed as the cathodic reaction. Once the zinc corroded to the steel substrate or at any uncoated areas, the steel would be protected but act as a cathode with hydrogen gas formation leading to embrittlement. Furthermore, investigations after rod breakages showed that the grouting was missing or incomplete in a number of the holes. The grouting is an essential part of the corrosion protection system. If moisture can build up in the grout ducts, then both general corrosion and hydrogen damage are likely. Proper quality control and inspection to ensure this crucial stage is carried out is absolutely essential.
Quality Assurance, Quality Control and Records
The ability to analyze the various issues that have arisen on this project is in part due to the records, along with the compliance and traceability requirements of Caltrans. Caltrans required their own inspection or auditing as well as quality control and detailed documentation by the relevant contractor. However, it appears from the problems noted above that some important inspection stages were missed and that critical problems were overlooked by both the quality control and Caltrans inspectors. This would appear to be due to lack of knowledge of the various processes that were being monitored. The best QA system is pointless if the actual QC technicians and inspectors do not understand the processes and key inspection stages.
Terminology
The investigations have highlighted a problem with terminology for failures involving hydrogen. Historically, it was thought that embrittlement and failures due to hydrogen would arise during steel making and fabrication and terms such as hydrogen embrittlement and hydrogen induced cracking were reserved for such failures. Similar failures arising due to the hydrogen produced by corrosion reactions were believed to result from a different failure mechanism and considered as a form of “stress corrosion cracking.”
Subsequent research has shown that the mechanism in both cases is the same, namely the presence of hydrogen embrittling high strength steel causing it to fail at a stress lower than it is designed to withstand. Now, the generally accepted term used to distinguish between the two forms (if necessary) is Internal and Environmental hydrogen embrittlement (IHE and EHE). The term stress corrosion cracking should be restricted to failure where active path metal corrosion assists in the formation of cracks, such as failure of certain stainless steels in chloride environments at above ambient temperatures. Stress corrosion cracking is rare in structural and fastener steels, even at these strength levels, at ambient temperatures. However, the term “stress corrosion cracking” is still used for the EHE, even by experts in the field. This confusion does not assist designers who may believe they have to consider hydrogen as an issue only during fabrication.
Lessons Learned
- When using high strength fasteners under significant loads, hydrogen embrittlement is a real possibility and all steps should be taken to minimize the risk.
- An upper limit on surface hardness of around Rockwell C 35 should be specified and QC and inspection of steel mechanical properties are critical.
- If possible, zinc coating, especially hot dip galvanizing, should be avoided for such fasteners and alternative methods of corrosion control used. This is to minimize the risk of environmental hydrogen embrittlement, especially in concrete environments.
- The grouting of such fasteners is critical and all steps must be taken to ensure that such fasteners are 100 per cent encapsulated in protective grout.
There will usually be a risk of hydrogen embrittlement using high strength fasteners so it is imperative that designers, contractors and inspectors are aware of all aspects of the material properties, heat treatment requirements, fabrication issues, corrosion protection and installation of such items.