Stainless steels are steels with a minimum of 10% chromium. They gain their resistance to corrosion from a thin, tenacious surface layer of chromium oxide. If the oxide layer is physically damaged, there is rapid regeneration of the layer, thus preserving the corrosion resistance. However, a chemical environment that can disrupt this layer can initiate corrosion. Consequently, stainless steel is highly resistant to atmospheric corrosion, but not immune to corrosion in all environments. (Atmospheric corrosion is examined in detail in the article The 5 Factors of Atmospheric Corrosion.)
Classes of Stainless Steel
Stainless steels fall into several general classes: austenitic, ferritic, martensitic, duplex and precipitation hardened (PH). The distinction between each class is based primarily on the predominant phase present in the stainless steel as determined by the major alloying elements.
The major alloying elements in stainless steels are chromium and nickel. Chromium primarily provides corrosion resistance and additional strength. Nickel provides strength and some corrosion resistance. Minor alloying elements include manganese, carbon and molybdenum. Manganese is present in steels in small quantities, but at higher concentrations it stabilizes austenite and partially replaces nickel in the 200-series steels. Carbon is largely an impurity in austenitic steel, but it is a strengthening element in ferritic and martensitic steel, much as it is for carbon and low-alloy steel. Molybdenum provides additional strength and resistance to chloride pitting. Other elements, such as titanium or niobium, serve other purposes specific to the application for which the alloy was developed.
Types of Stainless Steel
400-series steels were the first versions of stainless steel. They include the ferritic and martensitic grades that contain only chromium as a major alloying element, making them less expensive than austenitic grades. They are magnetic, generally more resistant to chloride attack than 300-series alloys, and some grades may be strengthened by heat treating.
Type 410 contains about 12% chromium. The ability to strengthen this alloy by heat treating to form martensite makes it a martensitic grade. Its low chromium content provides modest corrosion resistance. Given enough time, exposure to weather will cause it to rust. Type 430 is a ferritic grade that contains about 17% chromium. It cannot be strengthened by heat treating. 400-series stainless steels are generally more resistant to chloride attack than 300-series.
With sufficient quantities of nickel, stainless steel remains austenite at room temperature, creating the austenitic steels. They are nonmagnetic and cannot be heat treated for through hardening, like carbon steels, because the phase transformation to martensite does not occur in these alloys. The primary reason for their use is their superior resistance to corrosion in the atmosphere and aggressive chemical environments compared to 400-series.
300-series alloys contain chromium and nickel, and are the most popular austenitic grades. Types 301 and 304 are the most common alloys in use and are for general use. They contain 18% chromium, 9% to 10% nickel, and up to 0.15% carbon (301) or 0.08% carbon (304) as an impurity. Other 300-series alloys are modified versions of these alloys to achieve specific properties. Type 316 contains 2–3% molybdenum to improve the resistance to corrosion in chloride-containing environments. Types 304L, 316L and other L-grades contain reduced carbon, less than 0.03%, to avoid microstructure changes during welding and other thermal processes, which can damage the corrosion resistance. This detrimental change is known as sensitization. Types 321 and 347 contain small amounts of titanium and niobium, respectively, to prevent sensitization. They are capable of service at elevated temperatures, while the L-grades are intended to resist sensitization during fabrication. The image below shows the austenite grains in a 304 alloy.

Austenite grains in a 304 alloy. The particles on the grain boundaries are chromium carbides.
200-series steels, also austenitic, have manganese substituted for some of the nickel as a cost-saving measure. Grade 201 contains about 17% chromium, 6.5% manganese and 4% nickel. It has corrosion resistance similar to 301.
Precipitation hardening (PH) steels are strengthened by heat treating to form precipitates, as well as by martensite formation. They can be strengthened to higher hardness than 400-series grades by an aging method similar to that of aluminum alloys. 17-4 PH and 17-7 PH steels contain 17% chromium and 4% or 7% nickel, respectively. Minor alloying elements can include copper, titanium and niobium, and others.
Duplex stainless steels allow savings in material costs in corrosive applications such as chemical processing, including chloride and sulfur-bearing environments. They consist of a mixture of austenite and ferrite in roughly equal proportions. Duplex stainless steels are subdivided into lean, standard, super or hyper-duplex based on the quantity of alloying elements. Duplex stainless steels contain more chromium and less nickel than 300-series and typically include nitrogen as an additional austenite stabilizer and molybdenum for corrosion resistance. (Related reading: A Look at High Nitrogen Stainless Steels.) 2205 (22% chromium, 5% nickel and 3% molybdenum) is a common standard duplex stainless steel, and 2507 (25% Cr, 7% Ni plus 4% Mo) is a common super-duplex steel. The micrograph below shows a duplex stainless steel.
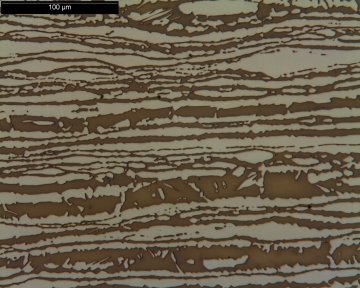
Micrograph of a duplex stainless steel. The brown colored phase is ferrite and the light colored phase is austenite. (Courtesy of Charles Young, P.E.)
How to Select the Proper Grade of Stainless Steel
The broad array of stainless steels available provides a vast portfolio of capabilities. However, each alloy has distinct advantages and disadvantages. When selecting a grade of stainless steels available, it is important to consider how components will be fabricated and joined together, the specific environment to which it will be exposed and the considerations common to other alloys such as mechanical requirements and cost.
More information about stainless steels is in "ASM Specialty Handbook: Stainless Steels" by J.R. Davis and "ASM Metals Handbook Volume 1: Properties and Selection of Irons, Steels, and High-Performance Alloys". This article was written with the assistance of Charles Young, P.E.
***
The article and images previously appeared at https://www.imetllc.com/introduction-stainless-steel/. Reprinted with permission. Copyright Industrial Metallurgists, LLC.